Quality Measures

Dr. Jorge
The RISE registry is used to track a variety of quality measures for clinical care of patients with rheumatic diseases. “Clinicians can view the RISE dashboard to track how they are doing in terms of meeting those quality measures,” explains Dr. Jorge. “Previously, there were no lupus-specific quality measures, but it is important to improve quality care for patients with lupus.” Through this project, she and Dr. Bartels convened a 17-member panel of rheumatologists, nephrologists, patients and experts in quality measure development, with the goal of selecting three key measures.
Using a modified Delphi process, the panel began by reviewing relevant scientific literature in North America and Europe from 2000–2020 and synthesizing the evidence. They identified 57 possible quality constructs, which they narrowed to 15. “We created if-then statements for the 15 and summarized supporting evidence regarding the public health impact,” Dr. Bartels says. Through voting, they winnowed the list to three, and finally vetted them with patients to ensure they felt the measures would have clear patient benefit. The process and measures are detailed in a manuscript published in 2023.3
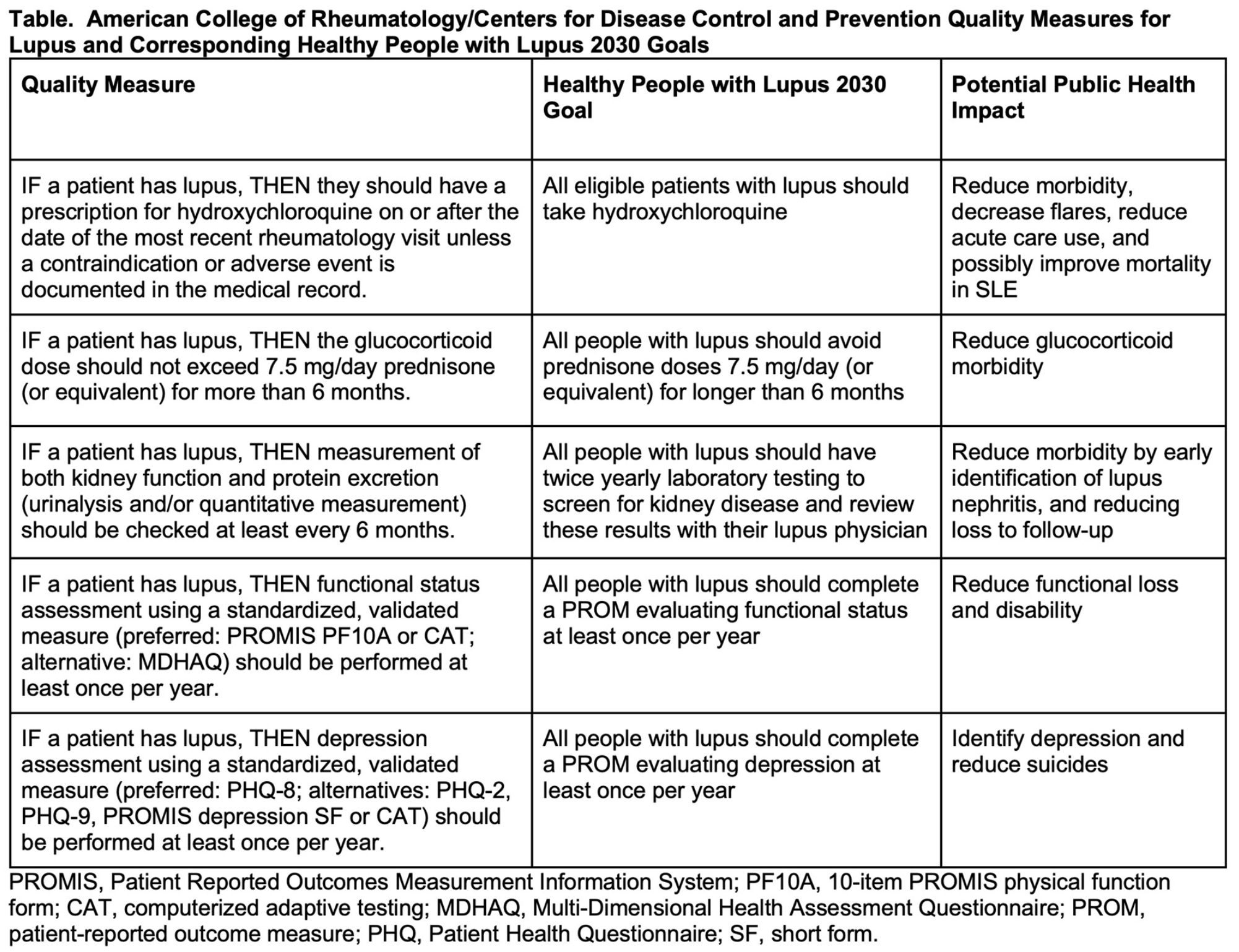
The three selected quality measures for SLE are:
- Hydroxychloroquine use for all patients without contraindications;
- Limits on the dose and duration of glucocorticoids not to exceed 7.5 mg/day of prednisone for longer than six months; and
- Regular kidney monitoring every six months to screen for and monitor lupus nephritis.
Regarding hydroxychloroquine, there are “good data showing that this drug reduces new organ involvement, cardiovascular disease and kidney disease,” Dr. Bartels says. “But when we talked to our patient advocates, who are very well informed and active in the lupus community, they actually had not had a conversation with their own doctors about how this one simple medicine can help them live longer. That highlighted to us that we need to make that message loud and clear.”
The second measure acknowledges that many adverse outcomes, including early stroke and early heart disease, are complications of glucocorticoids, as well as lupus itself. The measure’s recommendation to limit glucocorticoid use to no more than 7.5 mg daily by month six is consistent with EULAR and ACR guidelines, Dr. Bartels notes. “In fact, subsequent to our Arthritis Care & Research publication, EULAR is now recommending patients receive less than 5 mg. That may be a future benchmark for us, but for now our measure is staying at 7.5 mg, with the idea that patients and their doctors work together to switch to the steroid-sparing medicines that are now available.”