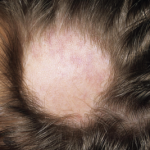
Xing and colleagues then investigated whether their therapeutic approach would be effective in human patients. They gathered preliminary data from a clinical trial using the JAK1/2 inhibitor, ruxolitinib, which has been approved by the U.S. Food and Drug Administration for the treatment of myelofibrosis. Three patients who had received oral ruxolitinib achieved near-complete hair regrowth after treatment. The results suggest that inhibiting JAK1 and JAK2 may be useful in the treatment of AA. The data are also consistent with the idea that CD8+NKG2D+ T cells are responsible for the autoimmune attack of hair follicles seen in patients with AA.
The researchers go on to suggest that a topical JAK1/2 and JAK3 inhibitor may be beneficial for the treatment of AA. However, Drs. Divito and Kupper note in their editorial that a topical JAK inhibitor with sufficient skin penetration has not yet been developed. (posted 10/24/14)
Lara C. Pullen, PhD, is a medical writer based in the Chicago area.
References