Clinical Photography, Central Manchester University Hospitals NHS Foundation Trust, UK / Science Source
SAN DIEGO—Top researchers gathered for a review course at the start of the 2017 ACR/ARHP Annual Meeting in November to describe new research, their own treatment strategies and new ways of thinking about an array of rheumatic diseases. Here are the highlights:
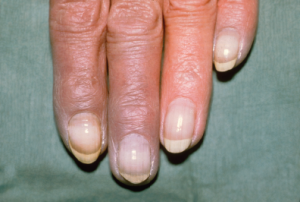
Raynaud’s & Other Digit Problems
When a patient walks into your clinic with blue fingers or toes, it can be a dramatic situation that requires quick action. Fred Wigley, MD, director of the Johns Hopkins Scleroderma Center in Baltimore, discussed these types of cases at the review course. He offered lessons on how this problem comes about, how to discern the cause and what to do about it.
Under normal conditions, the vessels regulating temperature subtly shift blood flow throughout the body to maintain the right temperature in the body’s core. The skin has an incredible capacity for this blood flow, able to accommodate 60% of the cardiac output. These thermal regulatory vessels are controlled by alpha 2 receptors that pick up norepinephrine from the sympathetic nervous system that can be triggered by skin cooling or a central body chill. Then the shunt contracts and restricts flow to the blood surface.
The vessels that provide nutritional blood flow have independent and distinct roles from the thermal regulatory vessels, or AV shunts. “The reason for that is the endothelial layer on the arterial flow and to the nutritional system maintains vasodilation to counteract the constrictive process,” Dr. Wigley said. This distinction “is important when we talk about danger” to the digits.
Patients presenting with Raynaud’s phenomenon and cold ischemic changes of the nose almost always have a circulating factor (e.g., cryoglobulines, cryofibrinogens) as a cause. “If you have ischemic lesions, you don’t have primary Raynaud’s,” Dr. Wigley said. Primary Raynaud’s is a symmetrical process involving all the fingers and the toes, with no ischemic lesions, sparing of the thumb, normal nailfold capillaries on exam and usually a negative serology.
Acrocyanosis can mimic Raynaud’s. It’s a nonparoxysmal, persistent, painless, symmetrical cyanosis of the hands (and sometimes the feet and knees) caused by vasospasm of the small vessels of the skin. Causes of secondary Raynaud’s include scleroderma, systemic lupus erythematosus (SLE), carpal tunnel syndrome, vibration, frostbite and certain drugs, including Ritalin.
One way to tell whether a patient with “very scary blue fingers” simply has cold fingers or has critical ischemia is to push on them. If you see rapid reflow, “that’s reassuring that there’s no critical ischemia,” Dr. Wigley said. If the reflow is slow, “you’ve got to be worried they’re going to have a catastrophic event” associated with irreversible, compromised blood flow.
Another sign of critical ischemia: pain. “The patient who comes in and says, ‘My whole finger hurts, not just the tip of the ulcer, but the whole finger,’ is in trouble.”

Dr. Rosenbaum
If a patient comes in with an ischemic foot and there’s a concern about big vessel disease, Dr. Wigley suggested elevating the foot. A normal foot will stay pink and won’t hurt, while an ischemic foot will turn white and hurt. “Patients with macrovascular disease like to keep their leg in a down position, so this [is something] you can do at the bedside,” he said.
He said clinicians should always consider macrovascular disease in cases of lower extremity digital ischemia.
Dr. Wigley shared his approach to managing Raynaud’s and digital ischemia. First, stop the aggravating factors, such as cold, trauma, stress and smoking. Then, for Raynaud’s with no ulcers, he’ll use a calcium channel blocker. For severe ischemia with ulcers, he’ll also use a phosophodiesterase-5 inhibitor, typically sildenafil. In even more severe cases, he’ll move to prostacyclin.
He also said not to forget protective agents: “We want to treat more than the vasospasm, we want to treat the underlying problem.”
For a finger or toe with acute ischemia, he said the first step is rest and warmth, followed by controlling pain with a digital nerve block (possibly lidocaine), then a vasodilator, such as a calcium channel blocker or prostaglandins.
‘Do you ever go to funerals of your patients? I went to her funeral.’ —Dr. Petri
Red Eyes & Rheumatic Disease
James Rosenbaum, MD, chair of the arthritis and rheumatic diseases division at Oregon Health & Science University in Portland, Ore., and chief of ophthalmology at the Devers Eye Institute, also in Portland, said a list of rheumatic diseases that involve the eye is a long one: “It’s virtually everything we see in our practice, and virtually every entity has a prototypic ocular manifestation.” And yet, he said, “My sense is rheumatologists are a bit intimidated by the eye.”
An important initial tool to remember, he said, is the acronym RSVP: redness that persists, sensitivity to light, visual change and pain. “If the patient is experiencing those things, that patient needs an ophthalmic evaluation,” he said.
Dryness remains the most likely cause of eye redness. That’s followed by episcleritis and scleritis, although those account for only 1% of cases of redness in these patients.
Dry Eye
About half of patients with rheumatoid arthritis (RA) have dry eye, as well as about half of lupus patients. “As rheumatologists, I’m convinced you can help your patients with dry eye,” Dr. Rosenbaum said.
The first step: Assess the patient’s medications. Antihistamines and medications for sleep, pain or depression all contribute to dry eye. “Those medicines interfere with our ability to measure tear production,” he said.
The differential for dry eye includes autoimmune disease, aging, effects of medications, HIV, surgery consequences, alcoholism, anxiety and Meibomian gland disease.
A humidifier in the bedroom, or even just a pan of water, can help. And replacing tears is more difficult than you might think. “Tear film is complicated,” Dr. Rosenbaum said, with water from the lacrimal glands, mucus from Goblet cells and oil from the Meibomian glands. “If the balance is off, the tear isn’t right. And to replace the tear, we need all three components, which no one has quite mastered.” Many tear film replacements contain a preservative, which can be toxic to the cornea, so preservative-free artificial tears are better for those needing to use them frequently.
Autologous serum, in which patients go to a blood bank that makes a sterile serum, is “arguably the most soothing replacement we have right now,” Dr. Rosenbaum said.
Two medications interfere with the immune response and are FDA approved to treat dry eye: topical cyclosporine and lifitegrast, a small molecule that interferes with leukocyte function associated antigen 1 and intercellular adhesion molecule 1. “Both have minimal ability to increase tear production,” Dr. Rosenbaum said. “It shows how impoverished we are, really, in terms of our therapeutic armamentarium for dry eye.”
Scleritis & Episcleritis
Scleritis, another cause of redness, is associated with RA, vasculitis (especially granulomatosis with polyangiitis), inflammatory bowel disease and other autoimmune disorders. Episcleritis, which involves the episclera that sits on top of the eye’s sclera and is typically benign, is scratchy while scleritis is painful; is short-lived versus persistent; and, upon use of phenylephrine drops, involves vessel blanching compared to no blanching in scleritis.
When evaluating patients with scleritis, a thorough history is crucial, and Dr. Rosenbaum said ANCA testing can prove informative in cases without a specific systemic association identified.
His first step in treatment includes a brief trial of an oral NSAID, to which roughly 15–20% will respond. If that doesn’t help, he moves to oral corticosteroids, immunosuppressives and treatment of underlying disease with biologics. He said he avoids periocular steroids because in a subset of patients the sclera can thin, bringing a risk of perforation.
His group found good results in a Phase I/II trial of rituximab in patients with noninfectious scleritis who haven’t responded to other treatments. “So it’s my go-to drug for scleritis [without] a systemic disease [and] that’s been refractory to other therapy,” he said.1
Suspected ANCA-Associated Vasculitis
Sharon Chung, MD, director of the Vasculitis Center at the University of California, San Francisco (UCSF), walked her audience through two cases that presented some of the complexities that can occur in suspected anti-neutrophil cytoplasmic antibody (ANCA)–associated vasculitis.
Patient 1
The first case involved a 59-year-old man undergoing treatment for Enterococcus endocarditis, with septic emboli with infarcts to the spleen, right kidney and brain. He was treated with ampicillin and ceftriaxone for a month, with negative blood cultures three days after starting the antibiotics. He began waiting for surgical repair of his aortic and mitral valves.
He then entered the clinic with small purpuric lesions on his legs and torso that were a week old, and with worsening shortness of breath and a cough that included blood. Diffuse alveolar hemorrhage was found on bronchoscopy the day before. His creatinine had risen from 0.9 to 3.0 mg/dL over the past month. He was PR3-ANCA positive.
He’d been on the anti-hypertensive hydralazine, and a chest CT showed diffuse bilateral parenchymal opacities with septal thickening and small pleural effusions, with no discrete nodules or cavities. A skin biopsy showed dermatitis with vacuolar change, and he was hypereosinophilic.
The disease possibilities included ANCA-associated vasculitis with pulmonary-renal syndrome, a drug-induced ANCA-associated vasculitis or just a set of unrelated events.
Dr. Chung said pulmonary-renal syndromes are caused by ANCA-associated vasculitis about 60% of the time, followed by p-ANCA combined with anti-GBM about 15% of the time, and anti-GBM alone about 4% of the time. Other causes, including SLE, account for the rest.
As for drug-induced ANCA, it can be brought on by anti-thyroid medications, antibiotics and other drugs such hydralazine and allopurinol. A 2014 study found ANCA positivity in patients with endocarditis, Dr. Chung noted.2
ANCA can also appear in nonvasculitic diseases. “It’s actually been detected in many autoimmune diseases,” she said.
In this case, doctors suspected drug-induced hypersensitivity syndrome. The patient’s hydralazine was stopped, and he was started on prednisone that tapered down over four weeks. He underwent mitral and aortic valve replacement, and his creatinine levels improved.
The final diagnosis: ANCA in the setting of endocarditis, with possible drug-induced vasculitis.
Patient 2
Another case involved a previously healthy 20-year-old woman who began suffering headaches, vomiting and nausea, and was found to have lost anterior pituitary function almost completely, with a biopsy showing necrotizing granulomatous hypophysitis. She was started on prednisone but returned three months later after three weeks of fevers, severe headaches, coughing (including hemoptysis) and other problems. She was found to have large, cavitating infiltrates on chest X-ray, and cavitary lesions with thick, irregular margins on chest CT. Her PR3-ANCA measured 686.
She was diagnosed with granulomatosis with polyangiitis (GPA) with an initial presentation of granulomatous hypophysitis.
CNS involvement in GPA appears in only about 1% of cases, Dr. Chung said, and among those cases, pituitary involvement isn’t common either. But the pituitary can be affected in three ways: in situ granulomatous infection, frank vasculitis of the pituitary vessels and extension of granulomatous inflammation from neighboring structures, such as the sinuses.
In the largest case series on the topic, nine of 819 patients with GPA were found to have pituitary involvement, with eight having active GPA at other sites at the time of diagnosis. The most common symptoms included headache and excessive thirst. Diabetes insipidus and hypogonadism were both found in seven of the patients.
All nine patients were treated with corticosteroids, with eight needing additional immunosuppression. Eight eventually needed hormone therapy.
GPA involving the meninges is more common—approximately 10% of cases, Dr. Chung said.
The woman in this case was treated with rituximab rather than the typical cyclophosphamide, and she now shows no evidence of active CNS or pulmonary disease, although her pituitary function has not recovered.
“Our gut-instinct, go-to medication in these situations tends to be cyclophosphamide. We’ve been using cyclophosphamide now for over 40 years in ANCA-associated vasculitis, and we know it works,” Dr. Chung said.
In this case, however, the woman was of child-bearing age, and there was a concern cyclophosphamide could affect fertility. Another consideration: Her pituitary function hadn’t recovered even after high doses of prednisone.
“The likelihood she would have pituitary recovery even with cyclophosphamide use was really low,” Dr. Chung said. “So we elected to treat her with rituximab.”
Assessing & Treating Axial Spondyloarthritis

Dr. Gensler
Lianne Gensler, MD, director of the Axial Spondyloarthritis Clinic at UCSF, offered tips on handling suspected cases of axial spondyloarthritis (axSpA). First, when you initially assess a patient and get their history, keep in mind their pain might not actually be where they say it is. For example, a patient might think their pain is in their hip, but then point to a location other than the hip.
On physical exam, you may not find anything immediately. But Dr. Gensler said to keep in mind that psoriasis is more common in the axSpA patient than the general population, so it’s worthwhile to look for signs of it in the nails and behind the ears.
Also, in the lab work-up, C-reactive protein (CRP) helps more than the sedimentation rate in suspected axSpA patients. And with imaging, X-ray is a good place to start, she said, but if it’s negative, move on to MRI of the sacrum, without gadolinium. She said you need to image only the sacrum—spine imaging doesn’t increase diagnostic yield and may produce findings that merely confuse.3
She emphasized the importance of imaging. MRI, she said, “does more than confirm your diagnosis. It will sometimes give you a different diagnosis. … It also gives you prognosis. We know that the higher burden of inflammation a patient has predicts response to therapy. We know if patients have structural change, including fat metaplasia or partial ankylosis, they’re more likely to go on to develop more damage.”
Although inflammatory back pain is the hallmark feature of axSpA (with the best predictors being nocturnal back pain and pain that improves with exercise), inflammatory back pain alone isn’t very useful as a predictor. “If you all you have is inflammatory back pain, then your probability of disease is only around 14%,” Dr. Gensler said.
Uveitis is the most prevalent extra-articular manifestation of axSpA, present about 25% of the time. Research has shown patients with anterior uveitis without a prior diagnosis of arthritis have spondyloarthritis, most commonly axSpA. “This is low-hanging fruit,” she said. “If a patient is referred to you from ophthalmology with HLA-B27 uveitis, you can almost guarantee you will find some form of arthritis in these patients.”
She issued some caution about a recent New England Journal of Medicine study on adalimumab for active noninfectious uveitis. “This study was not meant to include patients with anterior uveitis—the title doesn’t say that,” she said. “This isn’t meant to be used for patients with anterior uveitis alone, and in fact they were excluded from that study.”
The FDA has approved adalimumab only for patients with intermediate, posterior and panuveitis.
When treating to target, she said, choosing your target will prove just as important as how you’ll measure it. “For some patients, that target might be remission,” she said. “Other patients may have other reasons they won’t reach remission, so a low-disease activity endpoint is adequate. So, pick your measure and pick your endpoint, and then follow it.”
A trial on the IL-17A inhibitor, secukinumab, is the first of a biologic therapy in axSpA that includes patients who didn’t respond well to TNF inhibitors, Dr. Gensler noted. Patients showed a significantly greater response than placebo at 16 weeks, but some data suggest those with Crohn’s disease did worse on the drug than on placebo. IL-17A, she said, plays an important role in gut homeostasis.
She said that for now, she’d remain cautious with the drug in these patients, and her personal preference is to use 17A inhibitors only in those with axSpA who don’t have inflammatory bowel disease.
She said it remains somewhat unclear whether using secukinumab in patients predisposed to gut inflammation will make their inflammatory disease worse. “Long-term data will help unravel this,” she said.
Meniscus Tear & Adhesive Capsulitis

Dr. Senter
Carlin Senter, MD, associate professor of medicine at orthopedics at UCSF, gave rheumatologists guidance on navigating sports medicine cases. For starters, when evaluating a patient for a possible meniscus tear, she prefers to get a positive finding on at least three out of four tests before sending a patient for a knee MRI: the joint line tenderness test, the McMurray, the Thessaly and the squat test. The sensitivity of each of these tests is fairly low, so positive findings on multiple tests will yield more accurate results, she said.
The joint line tenderness test involves palpating between the femur and the tibia, looking for only joint line tenderness and not bony tenderness, which would instead indicate osteoarthritis (OA). The McMurray involves placing a hand over the patient’s knee with the knee flexed and turning the toes in and out. In the Thessaly, the patient stands on the affected knee, with their foot planted while rotating and the clinician looks for pain medially or laterally during the pivoting. On the squat test, the clinician simply looks for pain medially or laterally while the patient squats, holding on to the examiner if needed.
Dr. Senter commonly faces situations in which she must determine whether to recommend surgery for a patient with medial compartmental OA and a meniscus tear. Of the four most recent studies on this topic, three showed no significant difference between arthroscopic partial meniscectomy and physical therapy (PT) vs. PT alone.
One study limitation: The studies allowed crossover, and 30% of patients did switch from PT to surgery, she said. Crossing over was associated with a shorter duration of symptoms and a higher initial pain score.
“We’re still learning about who benefits from arthroscopic partial meniscectomy for a degenerative meniscus tear and who doesn’t,” Dr. Senter said. “But when you look at the literature broadly, it looks like most patients don’t benefit from arthroscopic meniscectomy for degenerative meniscus tears.”
She said it may be best to “think of a degenerative meniscus tear as part of the natural history of osteoarthritis. And so we tend to treat these patients as OA patients with a nonsurgical knee OA program to start.”
Indications for knee arthroscopy include an acute meniscus tear, a locked or locking knee, or a ligament tear.
Adhesive Capsulitis
When examining the shoulder, she said, the range of motion is the most crucial aspect. “The range of motion really helps me quickly triage what this patient has,” she said. “If the active range of motion is decreased, you really want to look at passive range of motion.”
If they’re both decreased, she said, the patient typically can only have arthritis of the glen humoral joint or adhesive capsulitis (i.e., frozen shoulder).
In frozen shoulder cases, an MRI doesn’t help, she said. “Adhesive capsulitis really is a clinical diagnosis,” she said. “Often an MRI will have findings in these patients who are most commonly age 40–60,” such as partial thickness rotator cuff tears, and the results could just bring confusion.
X-rays, meanwhile, can help to look at the glen humoral joint for arthritis.
Frozen shoulder can take two to three years to resolve, with a three- to nine-month freezing stage that hurts, a four- to 12-month frozen phase, with less pain but more stiffness, and a one- to three-year thawing stage of recovery. It usually resolves on its own.
Dr. Senter suggested diabetes screening in these patients. “One in five diabetics get a frozen shoulder,” she said. “It’s very common in diabetes.”
NSAIDs or steroid injections can manage pain, but they won’t alter the disease course, she said. Studies aren’t clear on whether physical therapy speeds recovery, but many patients seem to find it helpful, she said.
Doctors can recommend surgery in some cases, she said, but it’s fairly rare. “If a patient stays in the frozen stage up to a year, I tend to send those patients to our surgeon for arthroscopy. But the vast majority of patients really recover on their own.”
Fibrosing Lung Diseases
Sometimes the first sign of an autoimmune disease appears in the lung, said Paul Noble, MD, chair of pulmonary medicine at Cedars-Sinai Medical Center in Los Angeles. “I’ve seen patients I treated for what I thought was idiopathic pulmonary fibrosis … and their rheumatoid arthritis blossomed a decade later,” he said.
He offered some observations and tips for managing patients who present with fibrosing lung diseases that could be related to connective tissue diseases. First, he said, the question is always whether a manifestation in the lung is autoimmune related. “As a pulmonologist, we used to just give everybody steroids, so it didn’t really matter,” he said. “But now we know if you have a fibrosing lung disease for no good reason and we call it ‘idiopathic,’ we do more harm than good with immunosuppressive therapy. But if it’s an autoimmune disease, we can significantly change the natural history of the disease. So that’s the world I live in, trying to figure [it] out.”
Systemic diseases most often presenting with interstitial lung disease (ILD) include scleroderma and RA, but mixed connective tissue disease and polymyositis with antisynethase syndrome are also associated.
“Virtually none” of the patients who come to see him with respiratory problems and turn out to have an autoimmune disease complain of a lot of joint pain, though they sometimes have weakness of the joints. And routine labs for anti-nuclear antibodies, rheumatoid factor and sedimentation rate often prove inadequate for diagnosis, Dr. Noble said. “In my experience, I often find an underlying autoimmune disease when these three things are negative,” he said.
Because “it’s all about pattern recognition” when identifying lung diseases, it’s important to get a high-resolution CT. “High resolution is not what your patient gets when they go to the emergency room to rule out a pulmonary embolism,” he said. “High resolution means between 1 mm and 2 mm, and it’s really important to get that with the patients also breathing out. You want inspiratory and expiratory images.”
A ground-glass opacity on CT imaging (i.e., increased opacity but without obscuring of the underlying vessels) is often associated with good prognosis, he said. On imaging, “Where the infiltrates are is incredibly helpful,” Dr. Noble said. If the bottom of the lungs is the area most affected, it’s much more likely to be the idiopathic form.
When looking at labs to exclude systemic disease, an ESR of more than 100 excludes idiopathic pulmonary fibrosis. An ANA titre of at least 1:2560 is not idiopathic pulmonary fibrosis (IPF). Cyclic citrullinated protein (CCP) antibody is not the holy grail hoped for in distinguishing ILD caused by an autoimmune disease, because the false-positive rate for a middle-range CCP level remains unknown, he said.
Pirfenidone, an oral anti-fibrotic therapy, and nintendanib, a multi-kinase inhibitor, have been approved for IPF, but these drugs only slow the rate of loss of lung function, and they don’t eliminate scarring. In cases of connective tissue disease-related usual interstitial pneumonia (UIP), there’s no solid answer on whether patients should be treated with immunosuppression or an anti-fibrotic therapy. His approach, he said, is to treat them with immunosuppressive therapy. Then, if they progress, classify it as a case of UIP and try to get approval for anti-fibrotic treatment.
Antiphospholipid Antibody Syndrome
To convey the devastation potentially wrought by catastrophic antiphospholipid syndrome (CAPS), Michelle Petri, MD, MPH, director of the Johns Hopkins Lupus Center in Baltimore, told the story of a healthy, 17-year-old marathon runner who was put on oral contraceptives because of an ovarian cyst. Within weeks, she had congestive heart failure and fell into renal failure.
After treatment, she was able to come off a ventilator, with plans for a kidney transplant, but while in the operating room to have sections of infarcted bowel removed, a rare Mucormycosis fungal infection was found in her digestive tract—just the second case in more than 100 years of record-keeping at Hopkins. This prompted her doctors to halt immunosuppression, but within two days, the infection had eaten away her stomach. In a subsequent operation, she went into ventricular fibrillation arrest and couldn’t be resuscitated.
“Do you ever go to funerals of your patients?” Dr. Petri said. “I went to her funeral.”
Dr. Petri described the disease and a new way of thinking about it that could help lead to better treatment. CAPS, which accounts for just 1% of all antiphospholipid antibody syndrome, involves three or more organs, systems or tissues; manifests in a week; involves small-vessel occlusion; and requires lab confirmation of antiphospholipid antibodies. It has many triggers, including infections, drugs, SLE flares, obstetric causes and trauma. Many serious features appear in CAPS that rarely appear in regular APS, including acute respiratory distress syndrome, a comatose state, encephalopathy, congestive heart failure and cutaneous necrosis.
The initial therapy includes triple treatment with IV methylprednisolone, IV heparin and plasmapheresis or, if the patient isn’t stable enough for that, intravenous immunoglobulin.
A new treatment approach could be emerging, Dr. Petri said. “I want to introduce you to a whole new way of thinking about [CAPS],” she said. “I think there’s a new diagnosis out there. Rheumatologists will be part of this. And it’s called complementopathies.” Better known complementopathies include paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.
But, Dr. Petri said, CAPS is also a complementopathy involving the alternative pathway of complement being dysregulated. APS is largely due to autoantibodies that target beta-2 glycoprotein, a homologue of Factor H, a complement control protein.
Traditionally it’s taken weeks to wait to get back results to know whether a patient has a complementopathy. But researchers at Hopkins have developed a test called the modified Ham test that can give you the answer is just four hours. A treatment exists for complementopathies: eculizumab, although Dr. Petri acknowledged the exorbitant price tag of $500,000–750,000 a year.
Dr. Petri has anecdotal experience that rituximab could help. A middle-aged woman with APS who had cutaneous gangrene had failed triple therapy and was finally put on rituximab. The cutaneous gangrene stopped spreading. Then, after several days in a coma, she woke up.
Although she acknowledged it’s not evidence based, “when you have an experience like that, you become a true believer that when triple therapy fails, rituximab may play a role.” She said she would prefer eculizumab, but said clinicians “have to be reasonable about what we can actually get.”
For preventive treatment in patients with the lupus anticoagulant type of anti-phospholipid antibody, aspirin alone has proved insufficient. “But whatever is chosen needs to be as safe as aspirin,” Dr. Petri said, because over the course of 20 years patients will have a 50% chance of having a thrombotic event.
Hydroxychloroquine has been shown to reduce thrombosis risk by 50%, she said. Signaling downstream of complement activation—including activation of the tissue factor PAR-2 and neutrophils—has begun widening the range of potential therapies. They include vitamin D, which inhibits expression of tissue factor, and even statins, Dr. Petri said.
“We’re going to be much smarter in the future.”
Treatment Choice in RA

Dr. Kavanaugh
Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, was effusive in his assessment of the slate of options available to RA patients. “It’s been a very exciting time,” he said, but debate remains about how soon patients should be treated, he said.
Studies have found that early treatment of patients with undifferentiated arthritis has not proved effective at preventing the development of RA. But a 2017 study looked at patients with undifferentiated arthritis who were considered high risk using a score that included gender, age, stiffness, swollen joints and other factors. Researchers found that six of the 11 high-risk patients who were treated with methotrexate went on to develop RA, while all 11 in a placebo arm did. Another study found that treating similarly high-risk undifferentiated arthritis patients with a single dose of rituximab delayed the time to progression to RA.4,5
But this early treatment approach can be contentious, Dr. Kavanaugh said. “You’re overtreating some people to prevent it in others,” he said. “So it’s raised some issues that have affected the ethical consideration of study design.”
Although treating to target is “the ultimate idea,” the decision on which agents to use is “a little less” clear, he said. “Really, what we’ve learned in recent years is not bench to bedside as much as it’s been bedside to bench,” he said. “By using different targeted therapies across different diseases, we’ve learned more about those diseases. … With all the different agents we have, we’re learning more about how to use them, but we don’t have anything that will prospectively tell us.”
Biomarkers, demographic data, lab data and other potential predictors all seem to hold some promise for predicting treatment response. But when trying to figure out the best response at 24 weeks, “the strongest predictor is the response at 12 weeks,” Dr. Kavanaugh said.
He pointed to findings that suggest a deeper analysis could offer guidance. An analysis of data from the ADACTA trial found that individual biomarkers didn’t predict response very well, but serum biomarkers that correlate with immunopathophysiology within the synovium were predictive. Specifically, those with a higher myeloid pattern rather than lymphoid pattern in the serum performed better with TNF inhibition than IL-6 inhibition.6
“We’ve been looking in too simplistic a way,” Dr. Kavanaugh said. “We need systems biologics approaches to look at these complex volumes of data, and hopefully systems biology can help us get to something we can really bring to the clinic. But we’re just not there yet.”
Thomas R. Collins is a freelance writer living in South Florida.
References
- Suhler EB, Lim LL, Beardsley RM, et al. Rituximab therapy for refractory scleritis: Results of a phase I/II dose-ranging, randomized, clinical trial. Ophthalmology. 2014 Oct;121(10):1885–1891.
- Mahr A, Batteux F, Tubiana S, et al. Brief report: Prevalence of antineutrophil cytoplasmic antibodies in infective endocarditis. Arthritis Rheumatol. 2014 Jun;66(6):1672–1677.
- Ez-Zaitouni Z, Bakker PA, van Lunteren M, et al. The yield of a positive MRI of the spine as imaging criterion in the ASAS classification criteria for axial spondyloarthritis: Results from the SPACE and DESIR cohorts. Ann Rheum Dis. 2017 Oct;76(10):1731–1736.
- Burgers LE, Allaart CF, Huizinga TWJ, et al. Brief report: Clinical trials aiming to prevent rheumatoid arthritis cannot detect prevention without adequate risk stratification: A trial of methotrexate versus placebo in undifferentiated arthritis as an example. Arthritis Rheumatol. 2017 May;69(5):926–931.
- Gerlag D, Safy M, Maijer K, et al. Prevention of rheumatoid arthritis by B cell directed therapy in the earliest phase of the disease: The Prairi study. Annals of the Rheumatic Diseases. 2016;75:125–126.
- Dennis G Jr, Holweg CT, Kummerfeld SK, et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther. 2014;16(2):R90.