In Crohn’s disease and axial spondyloarthritis, Ruminococcus gnavus, a grampositive bacterium, is expanded in the gut and induces TNF expression by dendritic cells, which disrupts tight junction gut lining integrity by modulating occludin phosphorylation, releasing microbial products into the blood.19 Another microbe, Ruminococcaceae subdoligranulum, stimulates IgA production and a strong mucosal immune response that eventually becomes systemic. These cross-reactive IgA antibodies recognize RA self-antigens triggering joint pathology.20
A Western diet, rich in saturated fat and protein and low in fiber, alters the microbiome. This dysbiosis enhances the release of bacterial products, inducing the local production of proinflammatory cytokines, disrupting the epithelial barrier and affecting gut permeability.21,22
Leaky gut is associated with the release of antigens and microbial products into the bloodstream and activation of gut immune cells that circulate to joints and entheses and promote inflammation (see Figure 1). Thus, interventions focused to restore the balance in gut microflora and the integrity of epithelial barriers are appealing strategies to ameliorate disease severity and possibly onset in PsA.
Analyzing Synovial Fluid
Analysis of PsA synovial fluid and peripheral blood demonstrated the preferential accumulation of CD8+ T cells in the synovial fluid.23 The cells release high levels of IL-17 but they are not sequestered in RA synovial fluid. Subsequent studies of these cells revealed they are polyfunctional, releasing not only IL-17 and TNF, but also IFN-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF).
Moreover, some of these cells expressed T resident memory (TRM) cell markers. The TRM cells are sentinels in peripheral tissues with traditional α,β T cell receptors that respond rapidly to pathogens.24,25 The cells can also promote inflammation and have been implicated in the pathogenesis of several diseases, including psoriasis, mycosis fungoides and juvenile inflammatory arthritis.26-28
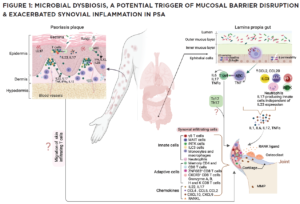
(CLICK TO ENLARGE) In this model, impaired pathogen immunity in PsA facilitates colonization of the skin and gut with bacteria that disrupt barrier integrity. Microbial products stimulate the release of chemokines (CCL2, CCL20), which draw innate immune cells including monocytes, macrophages, neutrophils, gamma delta T cells, innate lymphoid cells 3 (ILC), mucosal-associated invariant T cells (MAIT) and invariant natural killer T (iNKT) cells into target tissues. Innate and acquired immune cells secrete IL-17 and IL-23, inducing the proliferation of keratinocytes in the skin and the production of proinflammatory cytokines (IL-1, IL-6, TNF, IL-17) by resident synovial stromal cells. These cytokines trigger the secretion of additional chemokines responsible for the attraction and differentiation of CD4 and CD8 T cells in the skin and the joint. Tissue-infiltrating CD4 and CD8 T cells differentiate into Th17 or Tc17 cells, which release IL-17, a cytokine responsible for the generation of chemokines that attract neutrophils (CXCL1, CXCL2) and coordinate the migration of CXCR3+ T cells (CXCL9 and CXCL10). The presence of cycling, activated ZNF683+ resident memory CD4 and CD8 T cells in the synovia and skin of PsA patients release an array of cytokines. Expression of CD49a on the surface of synovial CD8 T cells indicates their potential bidirectional migration from or to the skin or the intestine. In summary, although Th17 cells are currently considered the primary drivers of PsA pathogenesis, novel findings support the participation of innate immune cells as a source of IL-17 and unexpectedly reveal the potential of resident memory CD8 T cells to mediate tissue inflammation and damage in PsA patients.


