 The etiology of psoriatic arthritis (PsA) is poorly understood but current evidence supports an interaction between genetic and environmental factors that coalesce to promote local tissue inflammation.1-3 The pivotal cytokines that underlie the local inflammatory response in a wide range of tissues are interleukin (IL) 23, IL-17 and tumor necrosis factor (TNF).4 The central contribution of these cytokines in the development of synovitis, enthesitis, dactylitis, psoriasis and axial disease is supported by discoveries in preclinical models and molecular studies of peripheral mononuclear blood cells.
The etiology of psoriatic arthritis (PsA) is poorly understood but current evidence supports an interaction between genetic and environmental factors that coalesce to promote local tissue inflammation.1-3 The pivotal cytokines that underlie the local inflammatory response in a wide range of tissues are interleukin (IL) 23, IL-17 and tumor necrosis factor (TNF).4 The central contribution of these cytokines in the development of synovitis, enthesitis, dactylitis, psoriasis and axial disease is supported by discoveries in preclinical models and molecular studies of peripheral mononuclear blood cells.
New Technologies, New Insights
Recent technologies—such as cytometry by time of flight (CyTOF), which uses detection of heavy metals conjugated to cell-specific antibodies, and single-cell RNA sequencing (scRNAseq), based on analysis of RNA expression in single cells—provide new insights into the key effector cell subsets in the skin and synovial fluid responsible for disease initiation and persistence. Moreover, emerging evidence implicates microbial dysbiosis in the development of joint inflammation. In this brief review, we describe these recent discoveries and provide a working model of PsA pathogenesis.
In one model, the critical events that set the stage for onset of PsA arise in the skin. This view is supported by the fact that in the majority of PsA patients, plaque psoriasis precedes onset of joint disease by several years and by the marked parallels in the immune responses present in the skin, joints and entheses.5 The progression from normal skin to the erythematous, well demarcated plaque with overlying scale is triggered by LL-37-driven activation of plasmacytoid dendritic cells (pDC), similar to the inciting events in the pathogenesis of systemic lupus erythematosus.6-8
The stimulation of pDC by the antimicrobial peptide LL-37 promotes the release of interferon-α (IFN-α) followed by the activation of T cells that circulate to the lymph node and return to the dermis where they take on the phenotype of Th1 and type 17 cells in response to interferon-γ (IFN-γ) and IL-23, respectively.9,10 Type 17 CD4+ and CD8+ cells secrete TNF, IL-17 and other cytokines. IL-17 released by T lymphocytes and innate immune cells stimulate the secretion of chemokines, cytokines and antimicrobial peptides by keratinocytes, amplifying the innate and adaptive inflammatory responses in the dermis and epidermis.11
Role of the Gut
In the paradigm outlined above, the inflamed skin is likely the tissue of origin for subsequent arthritis; however, emerging evidence in PsA, rheumatoid arthritis (RA), Crohn’s disease and osteoarthritis support the gut’s vital contribution to musculoskeletal inflammation.12-15 Thus, the origin of musculoskeletal inflammation may arise as a result of intestinal dysbiosis. Indeed, Scher et al. showed a low microbiome diversity in feces of PsA patients that favored local inflammation. Additional studies are underway to identify the immune-mediated pathways activated by the pathogens and bacterial products to induce systemic and joint immunity.16-18
In Crohn’s disease and axial spondyloarthritis, Ruminococcus gnavus, a grampositive bacterium, is expanded in the gut and induces TNF expression by dendritic cells, which disrupts tight junction gut lining integrity by modulating occludin phosphorylation, releasing microbial products into the blood.19 Another microbe, Ruminococcaceae subdoligranulum, stimulates IgA production and a strong mucosal immune response that eventually becomes systemic. These cross-reactive IgA antibodies recognize RA self-antigens triggering joint pathology.20
A Western diet, rich in saturated fat and protein and low in fiber, alters the microbiome. This dysbiosis enhances the release of bacterial products, inducing the local production of proinflammatory cytokines, disrupting the epithelial barrier and affecting gut permeability.21,22
Leaky gut is associated with the release of antigens and microbial products into the bloodstream and activation of gut immune cells that circulate to joints and entheses and promote inflammation (see Figure 1). Thus, interventions focused to restore the balance in gut microflora and the integrity of epithelial barriers are appealing strategies to ameliorate disease severity and possibly onset in PsA.
Analyzing Synovial Fluid
Analysis of PsA synovial fluid and peripheral blood demonstrated the preferential accumulation of CD8+ T cells in the synovial fluid.23 The cells release high levels of IL-17 but they are not sequestered in RA synovial fluid. Subsequent studies of these cells revealed they are polyfunctional, releasing not only IL-17 and TNF, but also IFN-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF).
Moreover, some of these cells expressed T resident memory (TRM) cell markers. The TRM cells are sentinels in peripheral tissues with traditional α,β T cell receptors that respond rapidly to pathogens.24,25 The cells can also promote inflammation and have been implicated in the pathogenesis of several diseases, including psoriasis, mycosis fungoides and juvenile inflammatory arthritis.26-28
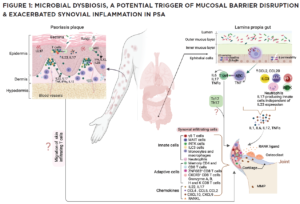
(CLICK TO ENLARGE) In this model, impaired pathogen immunity in PsA facilitates colonization of the skin and gut with bacteria that disrupt barrier integrity. Microbial products stimulate the release of chemokines (CCL2, CCL20), which draw innate immune cells including monocytes, macrophages, neutrophils, gamma delta T cells, innate lymphoid cells 3 (ILC), mucosal-associated invariant T cells (MAIT) and invariant natural killer T (iNKT) cells into target tissues. Innate and acquired immune cells secrete IL-17 and IL-23, inducing the proliferation of keratinocytes in the skin and the production of proinflammatory cytokines (IL-1, IL-6, TNF, IL-17) by resident synovial stromal cells. These cytokines trigger the secretion of additional chemokines responsible for the attraction and differentiation of CD4 and CD8 T cells in the skin and the joint. Tissue-infiltrating CD4 and CD8 T cells differentiate into Th17 or Tc17 cells, which release IL-17, a cytokine responsible for the generation of chemokines that attract neutrophils (CXCL1, CXCL2) and coordinate the migration of CXCR3+ T cells (CXCL9 and CXCL10). The presence of cycling, activated ZNF683+ resident memory CD4 and CD8 T cells in the synovia and skin of PsA patients release an array of cytokines. Expression of CD49a on the surface of synovial CD8 T cells indicates their potential bidirectional migration from or to the skin or the intestine. In summary, although Th17 cells are currently considered the primary drivers of PsA pathogenesis, novel findings support the participation of innate immune cells as a source of IL-17 and unexpectedly reveal the potential of resident memory CD8 T cells to mediate tissue inflammation and damage in PsA patients.
Also, unique environmental clues are critical for chemotaxis of immune cells to the synovial tissue. For example, CXCR3 expression by CD8 T cells and the local production of CXCR3 ligands (CXCL9, CXCL10) likely explain the considerable accumulation of CD8 T cells in the synovial tissue and fluid of PsA patients. Interestingly, tissue-resident CD8 T cell clusters express the skin/gut markers (ITGA1: CD49a), supporting their potential migration from the skin or gut to the synovium or, alternatively, moving from the synovium to the skin or gut (see Figure 1).29
The application of CyTOF and single-cell RNA sequence analysis identified three CD8 T cell subsets in PsA synovial fluid characterized by class II expression (human leukocyte antigen-DR [HLA-DR] low vs. high) and ZNF683—a gene classically expressed by tissue-resident memory T (TRM) cells.
The detection of proliferative markers like MK167 and STMN1 and the evidence of clonal expansion based on T cell receptor (TCR) α and ß sequencing in CD8 T cells provide evidence of local activation and suggest antigen-driven CD8 T cell activation and expansion inPsA synovial fluid. Also, expression of genes involved in the production of cytotoxic molecules (i.e., granzyme A, B, H and K) and chemokines (i.e., ccl4, ccl5) by HLA-DR high CD8 TRM cells was also identified.29
Thus, the findings generated from examination of synovial fluid reveal a predominant CD8 T cell response with clonal features and polyfunctional cells that release various cytokines critical for activation of stromal cells along with adaptive and innate immune cells in target tissue. Also, the capacity of CD8 T cells to produce chemokines facilitates recruitment of additional immune cells resulting in the amplification and persistence of local inflammation.
Studies in our lab, currently focused on understanding the functional heterogeneity of CD8 T cells and their migratory and functional characteristics in PsA patients with a humanized murine model, may lead to strategies that modulate their pathogenic functions and alleviate skin and musculoskeletal inflammation.
The studies outlined above demonstrated a dominant CD8 cell response in the synovial fluid but these cells expressed few activation markers. In contrast, monocytes in PsA synovial fluid showed a prominent activation profile. CyTOF analysis showed that monocytes and macrophages in synovial fluid produced significant amounts of CCL2, which is likely responsible for recruiting inflammatory monocytes to the inflamed synovia and osteopontin, a matricellular protein with multiple functions including upregulation of IL-1ß and modulation of signaling and chemoattractant pathways in monocytes, fibroblasts and bone.30-32
These results indicate that modulation of molecules orchestrating monocyte/macrophage migration or functions are also critical therapeutic targets in PsA.
Examining Psoriatic Tissue
The studies outlined above focused on cells in the synovial fluid but we do not know how well these findings reflect ongoing events in psoriatic synovial tissue. Ultrasound-guided needle biopsies permit the retrieval of high-quality synovial tissue that can be dissociated and analyzed by CyTOF and scRNAseq to provide information about the molecular profiles and subsets of resident and tissue infiltrating cells.
Newer technologies, however, provide much needed information on cell-cell interactions in target tissues. The application of novel high-throughput imaging technologies, such as spatial transcriptomics, CyTOF imaging, co-detection by indexing (CODEX) and multiplexed error-robust fluorescence in situ hybridization (MerFISH), generates data visualizing the strategic positioning, distribution and interactions of cells in target tissues.
These novel imaging technologies will provide vital insights into the diverse cell subsets along with their distribution and cellular interactions that, if adequately targeted, have strong potential to disrupt both skin and synovial disease pathways in PsA.
The efforts to apply the above technologies to the study of psoriatic skin, synovium and blood will soon be set into motion with establishment of the Accelerating Medicine Partnerships Autoimmune and Immune-Mediated Diseases (AMP AIM) in the U.S. and Health Initiatives in Psoriasis and Psoriatic Arthritis Consortium European States (HIPPOCRATES) in Europe and the United Kingdom.
These consortia, composed of partnerships between pharmaceutical companies, academic sites and, in the U.S., the National Institutes of Health, will assemble large well-phenotyped patient cohorts to collect skin and synovial biopsies coupled with blood samples.
These tissues will be analyzed with the technologies outlined above to better understand the function and cell-cell interactions of critical effector cell subsets and molecular pathways. It is anticipated that these studies will provide a highly detailed and integrative picture of the cellular and molecular landscape in PsA target tissues.
 Maria de la Luz Garcia-Hernandez, PhD, is a research assistant professor in the Division of Allergy, Immunology, and Rheumatology, Department of Medicine, University of Rochester, N.Y. She investigates the mechanisms of altered bone remodeling in PsA and the phenotype and contribution of synovial CD8 T cells during the transition of psoriasis to PsA using a humanized mouse model.
Maria de la Luz Garcia-Hernandez, PhD, is a research assistant professor in the Division of Allergy, Immunology, and Rheumatology, Department of Medicine, University of Rochester, N.Y. She investigates the mechanisms of altered bone remodeling in PsA and the phenotype and contribution of synovial CD8 T cells during the transition of psoriasis to PsA using a humanized mouse model.
 Christopher Ritchlin, MD, MPH, is a professor of medicine in the Allergy, Immunology and Rheumatology Division and the Center for Musculoskeletal Research at the University of Rochester Medical Center. His research team is focused on understanding psoriatic arthritis pathogenesis and the mechanisms that underlie the transition from psoriasis to psoriatic arthritis.
Christopher Ritchlin, MD, MPH, is a professor of medicine in the Allergy, Immunology and Rheumatology Division and the Center for Musculoskeletal Research at the University of Rochester Medical Center. His research team is focused on understanding psoriatic arthritis pathogenesis and the mechanisms that underlie the transition from psoriasis to psoriatic arthritis.
References
- Cafaro G, McInnes IB. Psoriatic arthritis: Tissue-directed inflammation? Clin Rheumatol. 2018 Apr;37(4):859–868.
- Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: Focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol. 2019 Mar;15(3):153–166.
- Talotta R, Atzeni F, Sarzi-Puttini P, Masala IF. Psoriatic arthritis: From pathogenesis to pharmacologic management. Pharmacol Res. 2019 Oct;148:104394.
- Schinocca C, Rizzo C, Fasano S, et al. Role of the IL-23/IL-17 pathway in rheumatic diseases: An overview. Front Immunol. 2021 Feb 22;12:637829.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009 Jul 30;361(5):496–509.
- Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007 Oct 4;449(7162):564–569.
- Lande R, Botti E, Jandus C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2014 Dec 3;5:5621.
- Lande R, Palazzo R, Gestermann N, et al. Native/citrullinated LL37-specific T-cells help autoantibody production in systemic lupus erythematosus. Sci Rep. 2020 Apr 3;10(1):5851.
- Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009 Jun;129(6):1339–1350.
- Tohyama M, Yang L, Hanakawa Y, et al. IFN-alpha enhances IL-22 receptor expression in keratinocytes: A possible role in the development of psoriasis. J Invest Dermatol. 2012 Jul;132(7):1933–1935.
- Ten Bergen LL, Petrovic A, Aarebrot AK, Appel S. Current knowledge on autoantigens and autoantibodies in psoriasis. Scand J Immunol. 2020 Oct;92(4):e12945.
- Marcolongo R, Bayeli PF, Montagnani M. Gastrointestinal involvement in rheumatoid arthritis: A biopsy study. J Rheumatol. Mar-Apr 1979;6(2):163–173.
- Eppinga H, Konstantinov SR, Peppelenbosch MP, Thio HB. The microbiome and psoriatic arthritis. Curr Rheumatol Rep. 2014 Mar;16(3):407.
- Rudan I, Sidhu S, Papana A, et al. Prevalence of rheumatoid arthritis in low- and middle-income countries: A systematic review and analysis. J Glob Health. 2015 Jun;5(1):010409.
- Datta P, Zhang Y, Parousis A, et al. High-fat diet-induced acceleration of osteoarthritis is associated with a distinct and sustained plasma metabolite signature. Sci Rep. 2017 Aug 15;7(1):8205.
- Kono H, Fujii H, Asakawa M, et al. Medium-chain triglycerides enhance secretory IgA expression in rat intestine after administration of endotoxin. Am J Physiol Gastrointest Liver Physiol. 2004 Jun;286(6):G1081–1089.
- Kono H, Fujii H, Ishii K, et al. Dietary medium-chain triglycerides prevent chemically induced experimental colitis in rats. Transl Res. 2010 Mar;155(3):131–141.
- Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015 Jan;67(1):128–139.
- Henke MT, Kenny DJ, Cassilly CD, et al. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):12672–12677.
- Chriswell M, Seifert J, Bloom M, et al. Plasmablast-derived autoantibodies from individuals at-risk for RA that target RA-relevant antigens are polyreactive with arthritogenic bacteria. Arthritis Rheumatol. 2021;73(suppl 10).
- Manzel A, Muller DN, Hafler DA, et al. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. 2014 Jan;14(1):404.
- Shi Z, Wu X, Santos Rocha C, et al. Shortterm Western diet intake promotes IL-23 mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol. 2021 Jul;141(7):1780–1791.
- Menon B, Gullick NJ, Walter GJ, et al. Interleukin-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol. 2014 May;66(5):1272–1281.
- Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013 May;14(5):509–513.
- Szabo PA, Miron M, Farber DL. Location, location, location: Tissue resident memory T cells in mice and humans. Sci Immunol. 2019 Apr 5;4(34):eaas9673.
- Clark RA. Resident memory T cells in human health and disease. Sci Transl Med. 2015 Jan 7;7(269):269rv261.
- Ferguson ID, Griffin P, Michel JJ, et al. T cell receptor-independent, CD31/IL-17Adriven inflammatory axis shapes synovitis in juvenile idiopathic arthritis. Front Immunol. 2018 Aug 6;9:1802.
- Turner DL, Goldklang M, Cvetkovski F, et al. Biased generation and in situ activation of lung tissue-resident memory CD4 T cells in the pathogenesis of allergic asthma. J Immunol. 2018 Mar 1;200(5):1561–1569.
- Steel KJA, Srenathan U, Ridley M, et al. Polyfunctional, proinflammatory, tissue-resident memory phenotype and functionof synovial interleukin-17A+CD8+ T cells in psoriatic arthritis. Arthritis Rheumatol. 2020 Mar;72(3):435–447.
- Yager N, Cole S, Lledo Lara L, et al. Ex vivo mass cytometry analysis reveals a profound myeloid proinflammatory signature in psoriatic arthritis synovial fluid. Ann Rheum Dis. 2021 Dec;80(12):1559–1567.
- Schuch K, Wanko B, Ambroz K, et al. Osteopontin affects macrophage polarization promoting endocytic but not inflammatory properties. Obesity (Silver Spring). 2016 Jul;24(7):1489–1498.
- Icer MA, Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. 2018 Sep;59:17–24.


