
courtesy ACR Daily News
CHICAGO—At Hot Topics in Myositis, a session at the 2018 ACR/ARHP Annual Meeting, three experts discussed new classification criteria for idiopathic inflammatory myopathies (IIM) and offered practical primers on overlap myositis conditions and inclusion body myositis (IBM).
New Myositis Classification Criteria
After a 10-year development process, the new EULAR/ACR Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups was published in 2017.1 These criteria reflect updated knowledge of the myositis disease spectrum and included 976 myositis cases and 624 comparators contributed by 47 rheumatology, dermatology, neurology and pediatric clinics worldwide, said Ingrid E. Lundberg, MD, PhD, a professor in the Division of Rheumatology at Karolinska Institute, Karolinska University Hospital, Sweden.
“Inflammatory myopathies are a heterogeneous group of diseases, now with several subdiagnoses that have been identified. There are a number of new autoantibodies that have been identified. It’s recognized that extramuscular organ involvement is frequently seen in patients,” said Dr. Lundberg.
The new criteria score variables in a case to determine the probability a patient has one of several IIM conditions. Variables include age of first symptom onset; clinical muscle symptoms, such as weakness; skin variables, such as heliotrope rash or Gottron’s papules; other clinical variables, such as dysphagia or esophageal dysmotility; laboratory variables, such as elevated serum creatine kinase (CK) and other muscle enzymes, as well as positivity for autoantibodies, such as anti-Jo-1; and muscle biopsy results. Each variable is weighted differently on the basis of whether the patient does or does not have muscle biopsy data. The criteria development committee recommended having 55% probability as a lower limit for classification, or a score of 5.5 without a muscle biopsy and 6.7 with biopsy. A patient with 90% probability is classified as a definite myositis case.
‘A normal CK does not exclude myositis.’ —Ingrid E. Lundberg, MD, PhD
A flow chart helps users subclassify patients with a confirmed IIM: They first consider the patient’s age of disease onset; patients under 18 years old are subclassified as having juvenile dermatomyositis (JDM) if they have a skin rash and juvenile myositis if they do not have a rash. Patients over 18 years old with a skin rash and muscle weakness are classified as having dermatomyositis (DM), and those without muscle weakness as having amyopathic DM.
Patients over 18 years old without the typical skin rash but with such clinical features as finger flexor weakness or rimmed vacuoles are classified with inclusion-body myositis, “whereas patients without these features end up in the group with polymyositis [PM],” said Dr. Lundberg. A free, online criteria calculator is available.
Advantages & Limitations
One subgroup of patients that may be classified with polymyositis may have immune-mediated necrotizing myopathy (IMNM), “which was hardly recognized when we started our project,” said Dr. Lundberg. Based on the 2016 European Neuromuscular Center workshop report, IMNM includes three subsets: anti-SRP myopathy, anti-HMGCR myopathy and antibody-negative IMNM.2 Anti-SRP and anti-HMGCR myopathy include patients with high CK, proximal muscle weakness and either anti-SRP or anti-HMGCR antibodies. The antibody-negative IMNM classification includes high CK; proximal muscle weakness; muscle biopsy features (including scattered necrotic fibers); different stages of necrosis, myophagocytosis and regeneration; and macrophages, but few lymphocytes.
Future EULAR/ACR classification criteria should include other missing myopathy subsets, including the antisynthetase syndrome, said Dr. Lundberg.3 These patients are a subset of either DM or PM, have anti-tRNA synthetase autoantibodies, and characteristically have myositis, interstitial lung disease, arthritis, fever and Raynaud’s phenomenon, she said.
When Dr. Lundberg and her colleagues tested the criteria using a cohort at their hospital, they found that compared with the Bohan and Peter criteria published in 1975,4 the new criteria show a higher percentage of definite cases (65% to 42%). However, 12.3% of cases could not be classified, compared with 7.10% under the previous criteria, “mostly because there is missing information in our registry, such as biopsy data,” she said.
“There are limitations with the new criteria. Of the new autoantibodies we have recognized, only anti-Jo-1 antibodies made it into the new criteria due to the low number of tested individuals for the other autoantibodies in the classification criteria cohort,” said Dr. Lundberg. “Patients with interstitial lung disease, but without muscle weakness and anti-Jo-1 antibodies, may not be classified as IIM with these criteria. Some new subtypes could not be identified,” such as IMNM and the antisynthetase syndrome. “We also need to validate the criteria in an external cohort with cases and comparators. This is in the pipeline.”
Diagnostic workup for a patient with possible myositis includes both clinical observation of specific skin rashes and muscle weakness, she said. The Manual Muscle Test 8 (MMT8) strength test often is used, but rheumatologists should add the Functional Index of Myositis test, which counts muscle repetitions in seven muscle groups, for more sensitive detection of weakness that takes fatigue into account, she said (see Tables 1).5
“In typical cases, you have signs of myopathy, but also have elevation of muscle enzymes. But a normal CK does not exclude myositis,” said Dr. Lundberg. “An EMG [electromyography] and muscle biopsy may be helpful, where you can see signs of inflammation, degeneration, necrosis and regeneration of muscle fibers.”
Patients who have anti-Jo-1 antibodies may present with different organ manifestations, and recent research shows other myositis-specific autoantibodies, including anti-MDA5, anti-NXP2 and anti-SAE, may present with skin rash without muscle weakness.6,7 Normal CK and even normal biopsy results do not exclude diagnoses like dermatomyositis. Additional tests, such as immune staining for MHC, magnetic resonance imaging (MRI) to look for edema or to help guide biopsy, EMG, muscle enzyme tests and testing for myositis-specific autoantibodies are helpful for diagnosis, as well as pulmonary function tests if patients also present with lung symptoms, she said.
Myositis Management
One proposed test to help manage myositis patients over time is the IMACS-IIM Disease Activity Core Set, which includes the patient’s and physician’s global assessment of disease activity on a visual analog scale (VAS), MMT8, physical function testing using the Health Assessment Questionnaire (HAQ), laboratory tests for serum activity of muscle enzymes, and assessment for the degree of extraskeletal muscle disease activity using the Myositis Disease Activity Assessment score, Dr. Lundberg said.8
“The 2017 EULAR/ACR classification criteria for myositis were a step forward to harmonize clinical research and clinical trials, but external validation is still needed. One way is to include the new autoantibodies,” she said. “For diagnosis, you still need to use your clinical evaluation, autoantibodies, MRI and muscle biopsy, and all are helpful depending on the different subtypes of myositis and different organ manifestations. As these patients often have multi-organ involvement, they should be managed with a multidisciplinary team, including a physical therapist, rheumatologist, dermatologist [and] pulmonologist.”
Overlapping Myositis

Table 1: MMT8
For a detailed guide on how to perform and score the MMT8, see Manual Muscle Testing Procedures for MMT8 Testing from the National Institute of Environmental Health Sciences.
Myositis may overlap with other rheumatic diseases, most often systemic sclerosis (SSc), but also rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), said Julie J. Paik, MD, MHS, assistant professor of medicine, Johns Hopkins Scleroderma Center and Johns Hopkins Myositis Center.
Researchers have attempted to describe and classify patients with overlap myositis (OM) conditions. A 2005 study found that up to 29% of subjects in a Canadian myositis cohort had an overlap syndrome.9 A 2014 study found that 23% of myositis patients in a Brazilian cohort had overlapping RA and 29% had overlapping SLE, she said.10 “This may include myositis with a few overlapping connective tissue disease features, such as Raynaud’s phenomenon, arthritis, ILD, and a few features of SSc and lupus,” or on the other hand, patients met strict criteria for both myositis and the overlapping condition such as scleroderma, she said.
Rheumatologists first published research on cases of OM in scleroderma 50 years ago.11,12 The prevalence of muscle weakness in these patients broadly varies—from 5% to 95%—in studies due to the lack of consensus classification criteria for overlap myositis, said Dr. Paik.
“Another reason for the broad range of the prevalence is that the patients have previously been assessed solely by having abnormal muscle enzymes alone or an abnormal EMG, MRI or muscle biopsy,” she said. “While previously underappreciated in scleroderma, it is now increasingly recognized that myopathy is a poor prognostic feature in systemic sclerosis.” In a large Canadian scleroderma cohort, mean survival time was much shorter in patients with an elevated CK of ≥200 or physician-reported history of myopathy. Other clinical features that impacted survival in this study were early diffuse SSc, topoisomerase 1 positivity, ribonucleoprotein positivity, ILD and male gender.13
To learn more about OM in SSc patients’ long-term outcomes, Dr. Paik and her colleagues at Johns Hopkins Scleroderma Center recently conducted a nested, case-control cohort of 1,718 scleroderma patients from 1990 to 2012, and identified 392 that had muscle weakness, as defined by a Medsger Muscle Severity Score of ≥1 (which indicates at least 4/5 strength on the MRC scale). The primary outcome of interest was the HAQ-DI, a validated patient reported outcome of disability.14
“The classic phenotype of these patients who had muscle weakness was that they were African American, have shorter disease duration and diffuse subtype. Weak patients also tended to have a higher burden of disease, with severe GI [gastrointestinal] involvement, a history of renal crisis, synovitis, more restrictive lung disease, higher RVSP [right ventricular systolic pressure], an ejection fraction of less than 50% and higher CK elevations. Not surprisingly, weak patients tended to have a higher HAQ-DI score,” said Dr. Paik.
The researchers also looked at these patients’ autoantibodies and found that anti-centromere positivity was somewhat protective. While both weak and non-weak patients had similar levels of most scleroderma-specific autoantibodies, weak patients tended to have a high anti-nuclear antibody (ANA) titer with a nucleolar pattern, she said.
As patients’ muscle severity scores rose, the researchers saw a matching rise in HAQ-DI scores. This connection was seen even when they controlled for confounders like age, gender, restrictive lung disease and synovitis.
In a subsequent study, they wanted to explore the etiology of muscle weakness in the 42 of 65 SSc patients who had had a muscle biopsy read at Johns Hopkins Scleroderma Center.15 In addition to analyzing the muscle biopsy characteristics of these patients, the electrophysiologic characteristics of these patients were also studied. On EMG about 48% of these patients had an irritable myopathy, and “surprisingly, we found that on nerve conduction results, 43.5% had an abnormal sural amplitude, meaning that they had no response when the sural nerve was stimulated, or if the sural amplitude was less than 5,” said Dr. Paik. In patients with a myopathy, researchers typically expect such “myopathic findings” on EMG only, not neuropathy. Both EMG and nerve conduction studies are performed on weak patients to determine the etiology of the weakness. When they reviewed these patients’ EMG data, they only expected to see signs of a muscle problem, not a nerve problem.
“When we looked at the individual muscle biopsy features, we found that necrosis was predominant, at 66%,” followed by inflammation, she said. Nearly half the weak patients also had acute neurogenic atrophy, which suggests that motor denervation may be a common pathologic process in weak SSc patients. “We then combined the aggregate individual histologic features and found that the most common histopathologic category was non-specific myositis (35.7%) and necrotizing myopathy (21.4%),” she said. Three patients had fibrosis only, which led the researchers to explore this unique histologic subset of scleroderma.
Fibrosing Myopathy
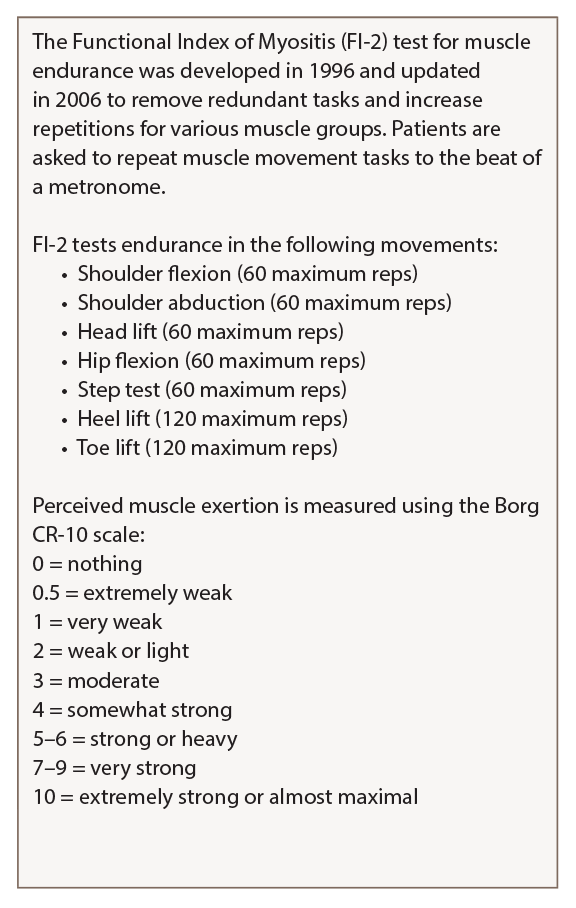
Table 2: Functional Index of Myositis
Karolinska Institute in Stockholm created a detailed guide on how to perform and score the FI-2 test that is available on the National Institute of Environmental Health Science website.
“After we found those three cases, we started to do a more systematic assessment of muscle weakness in all our scleroderma patients, and we started to see that, when we did biopsies, we started to see more of these patients,” said Dr. Paik. “So, we coined the term fibrosing myopathy, where we see fibrosis only and a lack of inflammatory infiltrates.” These patients’ fibrosis is primarily perimysial and endomysial, and thick, dense connective tissue encases the muscle fascicle, she explained.
In a 2017 study designed to further understand the clinical phenotype of fibrosing myopathy, the researchers compared these patients with those with an inflammatory myopathy, defined as inflammation and/or necrosis.16
“We saw that the fibrosing myopathy patients tended to be of the diffuse subtype, African American, and their duration of follow-up was really short—1.86 years vs. 5.93 years after a muscle biopsy, because most of them, unfortunately, were deceased.” They determined that fibrosing myopathy was associated with higher mortality. These patients also had elevated troponin, as well as lower CK and aldolase than those with inflammatory myopathy. They also identified two autoantibodies seen in fibrosing myopathy patients: anti-Scl-70 and anti-U3-RNP. The five deceased patients in the study all died of non-ischemic cardiomyopathy. Of the three patients who are still living, two are being treated with mycophenolate mofetil and intravenous immunoglobulin (IVIG), and doing well, and one is doing well on MMF alone, said Dr. Paik.
Another 2017 study determined that fibrosis was the predominant feature on muscle histopathology in 35 SSc patients, with 81% of SSc patients having fibrosis compared to 32% of patients with inflammatory myopathy.17
“Similar to our study, perimysial and endomysial fibrosis were the predominant finding,” said Dr. Paik. “They took it to another level, testing for TGF-β and type-I collagen, and they were also more prevalent in the patients who had scleroderma. In addition, because of the intense vasculopathy seen in patients with scleroderma, they also looked at VEG-F [vascular endothelial growth factor], and it was also increased in those patients with significant fibrosis.” Electron microscopy showed that these patients’ endothelial cell swelling exhibited microangiopathy and thickened basement membrane, as well as intense fibrosis in the endomysium and perimysium.
Patients with fibrosing myopathy have lower muscle enzymes, which may make it difficult for rheumatologists to diagnose this form of myopathy early, Dr. Paik said. While the optimal treatment algorithm for OM in SSc is unknown, “one thing is certain: We should not give high-dose steroids to our patients with scleroderma, because of the high risk of renal crisis. So we need to choose a steroid-sparing agent that can treat the skin and muscle,” she said.
Overlap Myositis in RA & SLE
In one of the first studies of OM in RA, conducted in 1984, 31 patients with active RA with peripheral joint inflammation were noted to have rheumatoid myositis, in which muscle biopsies showed interstitial, perivascular inflammation and myofiber necrosis.18 Since then, there has been a dearth of studies but in the clinical context, Dr. Paik said that “when we see patients with OM with RA, we’re really seeing patients with the antisynthetase syndrome.”
In a 2018 large cohort study of anti-Jo-1 patients, 64% of patients presented with symmetrical arthritis at disease onset. Anti-citrullinated protein antibody (ACPA) positive patients with the antisynthetase syndrome also tend to have higher swollen joint counts and more radiographic damage.19
Unfortunately, little data exist regarding myositis overlapping with SLE, said Dr. Paik. One NIH study in 1981 demonstrated that of 228 SLE patients, only 8% had muscle involvement, and aldolase may be a better marker than CK to assess for muscle involvement.20 A 1994 study evaluated muscle histopathology in 55 SLE patients and compared them to 26 controls and found these patients had a lymphocytic vasculitis as well as type-2 fiber atrophy, although this likely due to the fact that they were on corticosteroids.21
A flow chart helps users subclassify patients with a confirmed IIM: They first consider the patient’s age of disease onset; patients under 18 are subclassified as having juvenile dermatomyositis (JDM) if they have a skin rash & juvenile myositis if they do not have a rash. Patients over 18 with a skin rash & muscle weakness are classified as having dermatomyositis (DM), & those without muscle weakness as having amyopathic DM.
In terms of autoantibodies in OM, three autoantibodies have commercially available tests. They are anti-Ku antibodies, anti-PM-Scl and anti-RNP, said Dr. Paik, who co-authored a new study of 2,175 patients in the Johns Hopkins Myositis Cohort. This study was conducted to determine the clinical phenotype of OM patients with PM-Scl autoantibodies. They identified 949 patients who had had autoantibody testing, and of these, 41 had anti-PM-Scl compared to disease controls.22
“Anti-PM-Scl-positive patients had extensive extramuscular symptoms, which is not surprising,” she said. For example, these patients had more pronounced Raynaud’s phenomenon, mechanic’s hands and calcinosis than patients with other types of myositis in the study, including those with the antisynthetase syndrome, she said. They had less prevalent ILD than those with the antisynthetase syndrome, and also had more weakness in their arm abductors than their hip flexors. When muscle histopathology was evaluated in these 41 anti-PM-Scl-positive patients, intense perivascular inflammation was a common theme on muscle biopsy.
Anti-U3-RNP positivity is also higher in patients with overlap myositis. These patients tend to be African American, and have diffuse scleroderma, early-onset pulmonary hypertension, skeletal myopathy and severe GI disease, said Dr. Paik.23
Lastly, anti-Ku is seen in OM with SSc more often than in SLE, and some studies report that myositis in these patients tends to be mild with good response to treatment. These patients often have myofiber necrosis and inflammatory infiltrates, and a subset of anti-Ku-positive SSc patients with OM have a higher risk of severe ILD, she said.24
Inclusion Body Myositis
Exciting new data on inclusion body myositis (IBM) shows it may have a strong autoimmune element, said Andrew Mammen, MD, PhD, adjunct professor of neurology and medicine at the Johns Hopkins University School of Medicine and an investigator with the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
Based on his experience in diagnosing and treating patients with this rare disorder, Dr. Mammen said that one lesson is that “Twenty percent of patients who have inclusion body myositis will have no rimmed vacuoles on their biopsy. The absence of rimmed vacuoles on the biopsy does not exclude inclusion body myositis.25 Also, the classification criteria are only as good as the physical exam you bring to it. If you’re not finding the distal weakness, then you will come to the wrong diagnosis.”
Corticosteroids can decrease CK levels even when they do not treat the underlying myopathy, he said.
“I can treat many patients with genetic muscle disease or IBM with steroids, and their CK levels will go down, but they’re not getting any better. CK level changes do not always correlate with strength changes. It’s important to watch CK levels in IBM patients, but make sure we’re also looking at their strength,” he said.
IBM occurs in an estimated 12 in 100,000 people, and it is more common in males. Age of onset is typically after 50, with an insidious onset and slow progression. Patients have a unique, often asymmetric pattern of muscle weakness, frequently in the quadriceps, distal finger flexors, wrist flexors, triceps, ankle dorsiflexors and orbicularis occuli, and dysphagia is common.26
Several classification criteria have been published for IBM, the most recent in 2013, but all were based on expert opinion only, are complicated to use, and have high specificity, but low sensitivity, said Dr. Mammen, who co-authored diagnostic criteria for IBM with colleague Thomas E. Lloyd in 2014.27,28 They used machine-learning algorithms to test these data-derived criteria, which have 90% sensitivity and 96% specificity, he said. The criteria point to three features necessary for IBM diagnosis: finger flexion or knee extension weakness, endomysial inflammation on muscle biopsy, and invasion of non-necrotic muscle fibers or rimmed vacuoles on muscle biopsy.
“I like these criteria, because they just require a physical exam and basic immunohistochemistry. You don’t need any fancy staining,” he said.
IBM Pathogenesis
Tashatuvango / shutterstock.com
Is IBM a degenerative disease or an autoimmune myopathy? Features like MHC-1 upregulation and inflammatory infiltrates suggest the latter, Dr. Mammen said.
“On the other hand, you have these abnormal rimmed vacuoles and abnormal protein aggregates that suggest that IBM is a degenerative disease process, somewhat like Alzheimer’s disease of the muscle,” he said, noting this once seemed logical because patients’ muscle weakness does not respond well to treatment. “But my thinking has changed.”
Recent research suggests some IBM patients have a genetic predisposition to impaired autophagy, he said. Their rimmed vacuoles are enriched for proteins regulating autophagy and protein folding, including the protein FYCO1. Autophagy is a process that helps the body rid itself of abnormally folded proteins. A 2017 study showed that in whole exome sequencing, 11.3% of IBM patients had missense coding variants of FYCO1 compared to 2.6% of controls. FYCO1 plays a role in autophagosome transport, and these mutations may impair this process in patients with IBM and lead to impaired autophagy.29 IBM may have a primary autoimmune component: Evidence from a 1984 study shows that in IBM, cytotoxic CD8+ T cells invade the myofibers, said Dr. Mammen.30
“There are two populations of T cells. There’s a clonally diverse, noninvasive population of T cells, but there is also an autoinvasive, clonally restricted population invading these myofibers,” he said.31 “These clonal populations can persist for years in a single patient, and may be found in multiple muscles of the same patient, so you find these same T cell clones in multiple muscles.32 T cell clones are found both in the blood and muscle.33 All of this together suggests that there is some antigen-driven process in IBM that is leading to this clonal expansion of T cells.”
A recent study showed 58% of IBM patients have aberrant populations of circulating large granular lymphocytes and meet the diagnostic criteria for T-cell large granular lymphocytic leukemia (T-LGL).34
“These cells are clonal and stable, and they express various markers, including CD57, which is a marker for T cells undergoing persistent antigenic stimulation,” said Dr. Mammen. “These T cells have increased cytotoxic potential and resistance to apoptosis. They tend not to be killed by corticosteroids, which may be why these patients don’t respond to this treatment.” Patients who meet the T-LGL criteria tend to have more severe disease. LGL cells are more prevalent in the blood of IBM patients than in controls, and the CD87+ T cells invade a patient’s muscle fiber. “The more abnormal LGL lymphocytes you have in your blood, the more inflammation you have in your muscle.”
IBM’s phenotype differs from that of T-LGL in a few ways: More men have IBM than women—which is not true for T-LGL—and T-LGL features, such as neutropenia, are not seen in IBM, said Dr. Mammen.
“A reasonable hypothesis is that persistent, antigenic stimulation of T cells have sort of precipitated a neoplastic-like disorder, where you have a population of aggressive and resistant T cells, and it may explain why IBM is refractory to most immunosuppressive therapies like corticosteroids, because the T-LGL cells are also resistant to corticosteroids,” he said.
Anti-NT5C1a in IBM
Early immunohistochemical studies suggested qB cells are sparse in the muscles of IBM patients.35 However, more recent evidence shows that in gene expression profiling, immunoglobulin transcripts are abundant in their muscles, including the antibody-producing CD138+ plasma cells.36
“Where there are plasma cells and immunoglobulin transcripts, you expect to find an autoantibody,” said Dr. Mammen. Researchers identified an autoantibody, cytosolic 5’ nucleotidase 1A (NT5C1a), in patients with IBM. “This protein is abundant in skeletal muscle—it catalyzes nucleotide hydrolysis to nucleosides—but its function in muscle is not well understood.” Anti-NT5C1a is localized within rimmed vacuoles or around perinuclear areas of the IBM muscle.37 Patients with IBM who have NT5C1a antibodies tend to have more severe disease.38
Dr. Mammen proposed a speculative model of IBM pathogenesis based on genetic susceptibility toward autophagy and a strong autoimmune component.
“Maybe some patients with IBM have this genetic susceptibility to a myodegenerative process. Maybe this FYCO1 variant makes them less able to handle misfolded proteins. As the patients age, they have abnormal protein accumulation that causes damage to the muscle and cellular stress, which causes more abnormal protein accumulation,” he said. “Then, I could imagine that the damage to the muscle in patients who have the right immunogenetic predisposition—they’re male, maybe they have the right environmental triggers—these things come together and cause the initiation of autoimmunity.” Autoreactive T cells and autoantibodies come back to do more muscle damage, he said.
There are no approved treatments for IBM and most clinicians don’t believe immunosuppression has a significant sustained effect, so resist the urge to use immunosuppressants in these patients, said Dr. Mammen. “We need to develop drugs to deplete aggressive CD57+ and cytotoxic T cells.”
Exercise, such as low-impact aerobics, stretching and endurance exercises,
and physical therapy are helpful recommendations for these patients, he concluded. He recommended foot to ankle orthotics for patients with foot drop and training family members in the Heimlich maneuver to intervene when patients experience dysphagia.
Susan Bernstein is a freelance journalist based in Atlanta.
References
- Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol. 2017 Dec;69(12):2271–2282.
- Allenbach Y, Mammen AL, Benveniste O, et al. 224th ENMC International Workshop: Clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October, 2016. Neuromuscul Disord. 2018 Jan;28(1):87–89.
- Witt LJ, Curran JJ, Strek ME. The diagnosis and treatment of antisynthetase syndrome. Clin Pulm Med. 2016 Sep;23(5):218–226.
- Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975 Feb 13;292(7):344–347.
- Alexanderson H, Broman L, Tollback A, et al. Functional index-2: Validity and reliability of a disease-specific measure of impairment in patients with polymyositis and dermatomyositis. Arthritis Rheum. 2006 Feb 15;55(1):114–122.
- Trallero-Araguas E, Grau-Junyent JM, Labirua-Iturburu A, et al. Clinical manifestations and long-term outcome of anti-Jo-1 antisynthetase patients in a large cohort of Spanish patients from the GEAS-IIM group. Semin Arthritis Rheum. 2016 Oct;46(2):225–231.
- Gunawardena H, Betteridge ZE, McHugh NJ. Myositis-specific autoantibodies: Their clinical and pathogenic significance in disease expression. Rheumatology (Oxford). 2009 Jun;48(6):607–612.
- Miller FW, Rider LG, Chung YL, et al. Proposed preliminary core set measures for disease outcome assessment in adult and juvenile idiopathic inflammatory myopathies. Rheumatology (Oxford). 2001 Nov;40(11):1262–1273.
- Troyanov Y, Targoff IN, Tremblay JL, et al. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: Analysis of 100 French Canadian patients. Medicine (Baltimore). 2005 Jul;84(4):231–249.
- Aguila LA, Lopes MR, Pretti FZ, et al. Clinical and laboratory features of overlap syndromes of idiopathic inflammatory myopathies associated with systemic lupus erythematosus, systemic sclerosis, or rheumatoid arthritis. Clin Rheumatol. 2014 Aug;33(8):1093–1098.
- Medsger Jr. TA, Rodnan GP, Moossy J, et al. Skeletal muscle involvement in progressive systemic sclerosis (scleroderma). Arthritis Rheum. 1968 Aug;11(4):554–568.
- Thompson JM, Bluestone R, Bywaters EGL, et al. Skeletal muscle involvement in systemic sclerosis. Ann Rheum Dis. 1969 May;28(3):281–288.
- Jung M, Bonner A, Hudson M, et al. Myopathy is a poor prognostic feature in systemic sclerosis: Results from the Canadian Scleroderma Research Group (CSRG) cohort. Scand J Rheumatol. 2014 Mar;43(3):217–220.
- Paik JJ, Wigley FM, Mejia AF, et al. Independent association of severity of muscle weakness with disability as Measured by the Health Assessment Questionnaire Disability Index in scleroderma. Arthritis Care Res (Hoboken). 2016 Nov;68(11):1695–1703.
- Paik JJ, Wigley FM, Lloyd TE, et al. Spectrum of muscle histopathologic findings in forty-two scleroderma patients with weakness. Arthritis Care Res (Hoboken). 2015 Oct;67(10):1416–1425.
- Paik JJ, Wigley FM, Shah AA, et al. Association of fibrosing myopathy in systemic sclerosis and higher mortality. Arthritis Care Res (Hoboken). 2017 Nov;69(11):1764–1770.
- Corallo C, Cutolo M, Volpi N, et al. Histopathological findings in systemic sclerosis-related myopathy: Fibrosis and microangiopathy with lack of cellular inflammation. Ther Adv Musculoskelet Dis. 2017 Jan;9(1):3–10.
- Halla JT, Koopman WJ, Fallahi S, et al. Rheumatoid myositis. Clinical and histologic features and possible pathogenesis. Arthritis Rheum. 1984 Jul;27(7):737–743.
- Meyer A, Lefevre G, Bierry G, et al. In antisynthetase syndrome, ACPA are associated with severe and erosive arthritis: An overlapping rheumatoid arthritis and antisynthetase syndrome. Medicine (Baltimore). 2015 May;94(20):e523.
- Gonzalez-Gay MA, Montecucco C, Selva-O’Callaghan A, et al. Timing of onset affects arthritis presentation pattern in antisynthetase syndrome. Clin Exp Rheumatol. 2018 Jan–Feb;36(1):44–49.
- Tsokos GC, Moutsopoulos HM, Steinberg AD. Muscle involvement in systemic lupus erythematosus. JAMA. 1981 Aug;246(7):766–768.
- Lim KL, Abdul-Wahab R, Lowe J, et al. Muscle biopsy abnormalities in systemic lupus erythematosus: Correlation with clinical and laboratory parameters. Ann Rheum Dis. 1994 Mar;53(3):178–182.
- De Lorenzo R, Pinal-Fernandez I, Huang W, et al. Muscular and extramuscular clinical features of patients with anti-PM/Scl autoantibodies. Neurology. 2018 Jun 5;90(23):e2068–e2076.
- Aggarwal R, Lucas M, Fertig N, et al. Anti-U3 RNP autoantibodies in systemic sclerosis. Arthritis Rheum. 2009 Apr;60(4):1112–1118.
- Rigolet A, Musset L, Dubourg O, et al. Inflammatory myopathies with anti-Ku antibodies: A prognosis dependent on associated lung disease. Medicine (Baltimore). 2012 Mar;91(2):95–102.
- Chahin N, Engel AG. Correlation of muscle biopsy, clinical course, and outcome in PM and sporadic IBM. Neurology. 2008 Feb 5;70(6):418–424.
- Lefter S, Hardiman O, Ryan AM. A population-based epidemiologic study of adult neuromuscular disease in the Republic of Ireland. Neurology. 2017 Jan 17;88(3):304–313.
- Rose MR, ENMC IBM Working Group. 188th ENMC International Workshop: Inclusion Body Myositis, 2–4 December 2011, Naarden, The Netherlands. Neuromuscul Dis. 2013 Dec;23(12):1044–1055.
- Lloyd TE, Mammen AL, Amato AA, et al. Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology. 2014 Jul;83(5):426–433.
- Güttsches AK, Brady S, Krause K, et al. Proteomics of rimmed vacuoles define new risk allele in inclusion body myositis. Ann Neurol. 2017 Feb;81(2):227–239.
- Engel AG, Arahata K. Monoclonal antibody analysis of mononuclear cells in myopathies. II: Phenotypes of autoinvasive cells in polymyositis and inclusion body myositis. Ann Neurol. 1984 Aug;16(2):209–215.
- Fyhr I-M, Moslemi A-R, Lindberg C, et al. T cell receptor β-chain repertoire in inclusion body myositis. J Neuroimmunol. 1998 Nov;91(1–2):129–134.
- Müntzing K, Lindberg C, Moslemi A-R, et al. Inclusion body myositis: Clonal expansions of muscle-infiltrating T cells persist over time. Scand J Immunol. 2003 Aug;58(2):195–200.
- Dimitri D, Benveniste O, Dubourg O, et al. Shared blood and muscle CD8+ T-cell expansions in inclusion body myositis. Brain. 2006 Apr;129(Pt 4):986–995.
- Greenberg SA, Pinkus JL, Amato AA, et al. Association of inclusion body myositis with T cell large granular lymphocytic leukaemia. Brain. 2016 May;139(Pt 5):1348–1360.
- Greenberg SA, Bradshaw EM, Pinkus JL, et al. Plasma cells in muscle in inclusion body myositis and polymyositis. Neurology. 2005 Dec;65(11):1782–1787.
- Larman HB, Salajegheh M, Nazareno R, et al. Cytosolic 5′‐nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann Neurol. 2013 Mar;73(3):408–418.
- Goyal NA, Cash TM, Alam U, et al. Seropositivity for NT5c1A antibody in sporadic inclusion body myositis predicts more severe motor, bulbar and respiratory involvement. J Neurol Neurosurg Psychiatry. 2016 Apr;87(4):373–378.

