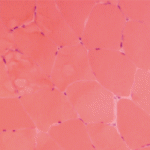
Inclusion Body Myositis
Exciting new data on inclusion body myositis (IBM) shows it may have a strong autoimmune element, said Andrew Mammen, MD, PhD, adjunct professor of neurology and medicine at the Johns Hopkins University School of Medicine and an investigator with the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
Based on his experience in diagnosing and treating patients with this rare disorder, Dr. Mammen said that one lesson is that “Twenty percent of patients who have inclusion body myositis will have no rimmed vacuoles on their biopsy. The absence of rimmed vacuoles on the biopsy does not exclude inclusion body myositis.25 Also, the classification criteria are only as good as the physical exam you bring to it. If you’re not finding the distal weakness, then you will come to the wrong diagnosis.”
Corticosteroids can decrease CK levels even when they do not treat the underlying myopathy, he said.
“I can treat many patients with genetic muscle disease or IBM with steroids, and their CK levels will go down, but they’re not getting any better. CK level changes do not always correlate with strength changes. It’s important to watch CK levels in IBM patients, but make sure we’re also looking at their strength,” he said.
IBM occurs in an estimated 12 in 100,000 people, and it is more common in males. Age of onset is typically after 50, with an insidious onset and slow progression. Patients have a unique, often asymmetric pattern of muscle weakness, frequently in the quadriceps, distal finger flexors, wrist flexors, triceps, ankle dorsiflexors and orbicularis occuli, and dysphagia is common.26
Several classification criteria have been published for IBM, the most recent in 2013, but all were based on expert opinion only, are complicated to use, and have high specificity, but low sensitivity, said Dr. Mammen, who co-authored diagnostic criteria for IBM with colleague Thomas E. Lloyd in 2014.27,28 They used machine-learning algorithms to test these data-derived criteria, which have 90% sensitivity and 96% specificity, he said. The criteria point to three features necessary for IBM diagnosis: finger flexion or knee extension weakness, endomysial inflammation on muscle biopsy, and invasion of non-necrotic muscle fibers or rimmed vacuoles on muscle biopsy.
“I like these criteria, because they just require a physical exam and basic immunohistochemistry. You don’t need any fancy staining,” he said.
IBM Pathogenesis
Tashatuvango / shutterstock.com
Is IBM a degenerative disease or an autoimmune myopathy? Features like MHC-1 upregulation and inflammatory infiltrates suggest the latter, Dr. Mammen said.
“On the other hand, you have these abnormal rimmed vacuoles and abnormal protein aggregates that suggest that IBM is a degenerative disease process, somewhat like Alzheimer’s disease of the muscle,” he said, noting this once seemed logical because patients’ muscle weakness does not respond well to treatment. “But my thinking has changed.”
Recent research suggests some IBM patients have a genetic predisposition to impaired autophagy, he said. Their rimmed vacuoles are enriched for proteins regulating autophagy and protein folding, including the protein FYCO1. Autophagy is a process that helps the body rid itself of abnormally folded proteins. A 2017 study showed that in whole exome sequencing, 11.3% of IBM patients had missense coding variants of FYCO1 compared to 2.6% of controls. FYCO1 plays a role in autophagosome transport, and these mutations may impair this process in patients with IBM and lead to impaired autophagy.29 IBM may have a primary autoimmune component: Evidence from a 1984 study shows that in IBM, cytotoxic CD8+ T cells invade the myofibers, said Dr. Mammen.30
“There are two populations of T cells. There’s a clonally diverse, noninvasive population of T cells, but there is also an autoinvasive, clonally restricted population invading these myofibers,” he said.31 “These clonal populations can persist for years in a single patient, and may be found in multiple muscles of the same patient, so you find these same T cell clones in multiple muscles.32 T cell clones are found both in the blood and muscle.33 All of this together suggests that there is some antigen-driven process in IBM that is leading to this clonal expansion of T cells.”
A recent study showed 58% of IBM patients have aberrant populations of circulating large granular lymphocytes and meet the diagnostic criteria for T-cell large granular lymphocytic leukemia (T-LGL).34
“These cells are clonal and stable, and they express various markers, including CD57, which is a marker for T cells undergoing persistent antigenic stimulation,” said Dr. Mammen. “These T cells have increased cytotoxic potential and resistance to apoptosis. They tend not to be killed by corticosteroids, which may be why these patients don’t respond to this treatment.” Patients who meet the T-LGL criteria tend to have more severe disease. LGL cells are more prevalent in the blood of IBM patients than in controls, and the CD87+ T cells invade a patient’s muscle fiber. “The more abnormal LGL lymphocytes you have in your blood, the more inflammation you have in your muscle.”
IBM’s phenotype differs from that of T-LGL in a few ways: More men have IBM than women—which is not true for T-LGL—and T-LGL features, such as neutropenia, are not seen in IBM, said Dr. Mammen.
“A reasonable hypothesis is that persistent, antigenic stimulation of T cells have sort of precipitated a neoplastic-like disorder, where you have a population of aggressive and resistant T cells, and it may explain why IBM is refractory to most immunosuppressive therapies like corticosteroids, because the T-LGL cells are also resistant to corticosteroids,” he said.