JGA / shutterstock.com
Immune checkpoint inhibitors (ICIs) are increasingly used to treat a variety of malignancies, leading to better cancer treatment outcomes and patient survival. However, the emergence of autoimmunity has been a major adverse event resulting from ICI use. Below, we describe a patient who experienced a flare of preexisting psoriasis and new-onset severe psoriatic arthritis after receiving nivolumab for treatment of his non-small cell lung carcinoma.
Case Presentation
A 61-year-old male presented at the rheumatology clinic complaining of new-onset joint pain and a worsening skin rash. The patient’s past medical history was significant for stage 4 non-small cell lung carcinoma diagnosed in November 2015. He was initially treated with carboplatin, gemcitabine and necitumumab, but he experienced tumor progression, necessitating discontinuation of that therapy. Nivolumab was started in September 2016. Eleven months following treatment initiation with nivolumab, the patient developed a flare of skin psoriasis and the onset of swelling and pain in multiple joints (see Figure 1).
He was referred to rheumatology, and on examination, synovitis was noted in the proximal interphalangeal and metacarpophalangeal joints as well as in his wrists and elbows. He had limited range of motion in his shoulders and hips. Extensive psoriatic plaques covered his upper and lower extremities, his abdomen and his frontal and temporal hairlines.
Inflammatory markers were elevated, and an anti-cyclic citrullinated peptide was negative. Rheumatoid factor was not checked. Hand X-rays were consistent with mild degenerative joint disease involving his bilateral distal interphalangeal and proximal interphalangeal joints, his first and second metacarpophalangeal joints and his left first metacarpal-trapezium joint space.
Nivolumab was discontinued by his oncologist one month after onset of his joint swelling and worsening psoriasis. The patient was started on 20 mg prednisone daily at his rheumatology evaluation. At follow-up, he reported 30–40% improvement in symptoms, but he continued to suffer from psoriasis, as well as pain and swelling in multiple joints.
Nivolumab was discontinued by his oncologist one month after onset of his joint swelling & worsening psoriasis.
Discussion
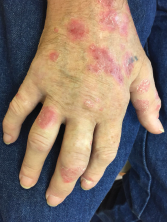
Eleven months following treatment initiation with nivolumab, a 61-year-old male developed a flare of skin psoriasis, as well as swelling and pain in multiple joints.
Nivolumab is a monoclonal antibody that inhibits programmed cell death protein 1 (PD-1) on the surface of T cells, thus preventing the protein’s interaction with programmed death-ligand 1 (PD-L1) on the tumor cell surface.1 Five PD1/PD-L1 checkpoint inhibitors (i.e., nivolumab, ipilimumab, pembrolizumab, tremelimumab and atezolizumab) have been FDA approved for the treatment of various malignancies, including melanoma, non-small cell lung carcinoma, renal cell carcinoma, Hodgkin lymphoma and urothelial carcinoma.1,2
Although several studies show great promise and survival benefits with these new medications, they also come with a potential disadvantage. Manipulating the off switch in the immune system could lead to autoimmune diseases and systemic inflammatory reactions, also known as immune-related adverse events (irAEs).1,3 Reported irAEs include rash, pneumonitis, hepatitis, colitis and hypothyroidism.2,4 Rheumatic irAEs are rare, and data regarding these side effects are limited.
In a case series reported by Calabrese et al., 15 patients were followed over an 18-month period. Of these, 13 patients with no preexisting autoimmune disease and two with an existing autoimmune disease reported several rheumatic irAEs, including inflammatory arthritis, sicca syndrome, polymyalgia rheumatica (PMR)-like symptoms and myositis.3
In the Calabrese study, the median time to onset of rheumatic irAEs was 7.3 weeks after initiating immunotherapy, with the exception of two patients who didn’t develop rheumatic irAEs until after more than a year. The longest time to onset was more than four years, in a patient receiving nivolumab for treatment of renal cell carcinoma who developed a PMR-like syndrome. One patient with preexisting psoriatic arthritis experienced an arthritis flare 2.8 weeks after initiating immunotherapy.3 Our patient described above had a prolonged time (11 months) to onset of his psoriasis flare and new psoriatic arthritis manifestations following the initiation of nivolumab.
The incidence of arthralgia secondary to nivolumab was reported at 5–16%, but the incidence of inflammatory arthritis remains unclear (probably due to poor documentation and a non-standardized coding system).5 In addition, some prescribers could have a lower suspicion of potential adverse effects of ICIs. Whether or not the incidence of rheumatic irAEs is increased in people with preexisting autoimmune disease remains unknown. In the case above, our patient did have a history of psoriasis, and we hypothesize he was more susceptible to rheumatic irAEs.
This case highlights an important emerging complication related to cancer immunotherapy with nivolumab, & the need for physicians to maintain a low threshold to suspect irAEs in patients on immunotherapy.
Prednisone has remained the initial treatment in rheumatic irAEs, although no treatment algorithms exist for the dose and duration of therapy.5 Some patients described above required steroid doses as high as 120 mg of prednisone daily, but others could be treated with a lower dose.5,6 Reports have been made of inflammatory arthritis irAEs persisting up to 15 months after the last dose of ICI. These patients required more aggressive therapy with tumor necrosis factor (TNF) inhibitors to control their symptoms.6
All 15 cases reported by Calabrese et al. required glucocorticoids, and three required a biological agent. Of those, 10 patients required their immunotherapy to be either permanently or temporarily discontinued.3 Given our patient’s advanced cancer, TNF inhibitors were not initiated. Methotrexate could have been an option for our patient, but he had a history of hepatitis C, and there are limited data on irAEs with methotrexate use.6 One of the patients reported by Calabrese et al. experienced a flare of psoriatic arthritis and was treated with apremilast with good response while remaining on immunotherapy.3 Apremilast was discussed as a potential therapy option with our patient, but unfortunately, he failed to follow up in the rheumatology clinic.
Conclusion
This case highlights an important emerging complication related to cancer immunotherapy with nivolumab, and it highlights the need for physicians to maintain a low threshold to suspect irAEs in patients on immunotherapy to achieve a timely diagnosis and initiate appropriate treatment.
Catherine Strahle, DO, is an internal medicine resident at the Department of Internal Medicine at the University of Cincinnati College of Medicine.
Nathalie E. Chalhoub, MD, is a rheumatology fellow at the Division of Immunology, Allergy and Rheumatology and an outcomes research fellow in the Internal Medicine Scholars Training for Academic Research (IMSTAR) program at the University of Cincinnati College of Medicine.
Avis Ware, MD, is a professor of medicine at the Division of Immunology, Allergy and Rheumatology at the University of Cincinnati College of Medicine, and the Rheumatology Clinical Director at University of Cincinnati Health.
Acknowledgment
The authors thank Christine L. Chhakchhuak, MD, for being part of the patient’s care and for all her efforts in coordinating the treatment plan with the patient’s oncologist.
References
- Abdin SM, Zaher DM, Arafa EA, et al. Tackling cancer resistance by immunotherapy: Updated clinical impact and safety of PD-1/PD-L1 inhibitors. Cancers (Basel). 2018 Jan 25;10(2). pii: E32.
- Kommalapati A, Sikder S, Tella S. Immune check point inhibitors in cancer therapy: Beware of “friendly fire” effect. Res Rev J Hosp Clin Pharm. 2017 Jul; 3(2):5–6.
- Calabrese C, Kirchner E, Kontzias A, et al. Rheumatic immune-related adverse events of checkpoint therapy for cancer: Case series of a new nosological entity. RMD Open. 2017 Mar 20;3(1):e000412.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015 Jul 2;373(1):23–34.
- Cappelli LC, Shah AA, Bingham CO. Immune-related adverse effects of cancer immunotherapy—implications for rheumatology. Rheum Dis Clin North Am. 2017 Feb;43(1):65–78.
- Cappelli LC, Gutierrez AK, Baer AN, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2017 Jan;76(1):43–50.



