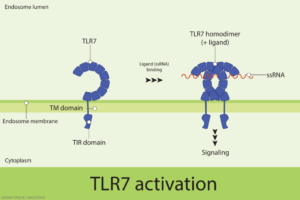
In 2022, an international group of researchers reported the seminal finding that a gain-of-function variant of a single-stranded ribonucleic acid (RNA) sensor, known as toll-like receptor 7 (TLR7), can cause human systemic lupus erythematosus (SLE).1 The paper in Nature showed that a newly described variant of TLR7, identified in a child with severe lupus, was sufficient to cause lupus in mice.
Heightened TLR7 activity in women, some studies have suggested, may help explain their immunity advantage over men through the increased recognition and binding of foreign RNA and initiation of a more robust immune response against perceived pathogens. But that relative advantage in the immune system’s defenses could come with a considerable downside. Activation of TLR7 by self-RNA, accumulating data suggest, can trigger pro-inflammatory pathways, such as an aberrant upsurge in type I interferon, a hallmark of rheumatic diseases, including lupus and Sjögren’s disease.
Researchers, however, hadn’t yet determined whether the binding of specific single-stranded RNA molecules to TLR7 drive that effect. Nor had they identified the basis of SLE’s dramatic sex bias, which causes the systemic autoimmune disease to affect ninefold more women than men.2
A recent study published in JCI Insight has helped partially fill in both of those blanks by implicating the long noncoding RNA of the X-inactive specific transcript (XIST) as a “female sex-specific danger signal underlying the sex bias in SLE.”3
The study’s lead author, Erika Darrah, PhD, an adjunct associate professor of medicine at Johns Hopkins University, Baltimore, notes that XIST was previously thought to play a protective role in lupus. The surprising new research, in fact, suggests the opposite. “Our study supports the idea that XIST actually plays a proinflammatory and disease-promoting role in lupus and—particularly relevant to the female susceptibility of lupus—at least partially explains why women are more likely to develop lupus than men,” Dr. Darrah says.
The multiple lines of evidence suggest that instead of acting as a regulator of gene expression, XIST may be a danger-associated molecular pattern, or DAMP, that sounds the alarm for the innate immune system. “That’s the paradigm-shifting premise here,” says co-author Brendan Antiochos, MD, an assistant professor of medicine at Johns Hopkins.
A Double-Edged Sword
In the developing female embryo, XIST is critical for silencing one X chromosome per cell; that process, known as X chromosome inactivation, helps normalize the dose of X-encoded genes between biological males and females. Importantly, the X chromosome encodes an abundance of immune-relevant genes, including TLR7.