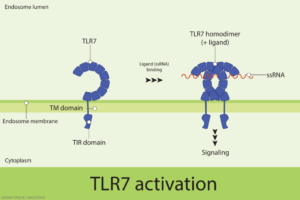
In 2022, an international group of researchers reported the seminal finding that a gain-of-function variant of a single-stranded ribonucleic acid (RNA) sensor, known as toll-like receptor 7 (TLR7), can cause human systemic lupus erythematosus (SLE).1 The paper in Nature showed that a newly described variant of TLR7, identified in a child with severe lupus, was sufficient to cause lupus in mice.
Heightened TLR7 activity in women, some studies have suggested, may help explain their immunity advantage over men through the increased recognition and binding of foreign RNA and initiation of a more robust immune response against perceived pathogens. But that relative advantage in the immune system’s defenses could come with a considerable downside. Activation of TLR7 by self-RNA, accumulating data suggest, can trigger pro-inflammatory pathways, such as an aberrant upsurge in type I interferon, a hallmark of rheumatic diseases, including lupus and Sjögren’s disease.
Researchers, however, hadn’t yet determined whether the binding of specific single-stranded RNA molecules to TLR7 drive that effect. Nor had they identified the basis of SLE’s dramatic sex bias, which causes the systemic autoimmune disease to affect ninefold more women than men.2
A recent study published in JCI Insight has helped partially fill in both of those blanks by implicating the long noncoding RNA of the X-inactive specific transcript (XIST) as a “female sex-specific danger signal underlying the sex bias in SLE.”3
The study’s lead author, Erika Darrah, PhD, an adjunct associate professor of medicine at Johns Hopkins University, Baltimore, notes that XIST was previously thought to play a protective role in lupus. The surprising new research, in fact, suggests the opposite. “Our study supports the idea that XIST actually plays a proinflammatory and disease-promoting role in lupus and—particularly relevant to the female susceptibility of lupus—at least partially explains why women are more likely to develop lupus than men,” Dr. Darrah says.
The multiple lines of evidence suggest that instead of acting as a regulator of gene expression, XIST may be a danger-associated molecular pattern, or DAMP, that sounds the alarm for the innate immune system. “That’s the paradigm-shifting premise here,” says co-author Brendan Antiochos, MD, an assistant professor of medicine at Johns Hopkins.
A Double-Edged Sword
In the developing female embryo, XIST is critical for silencing one X chromosome per cell; that process, known as X chromosome inactivation, helps normalize the dose of X-encoded genes between biological males and females. Importantly, the X chromosome encodes an abundance of immune-relevant genes, including TLR7.
After birth, some scientists have hypothesized that XIST could play a protective role against immune diseases, such as SLE, by silencing or dampening expression of X-encoded, pro-inflammatory genes. Dysregulation of XIST, they reasoned, may reactivate some of those genes and aid the development of autoimmunity.
XIST, in fact, is necessary to limit the expression of SLE-linked TLR7, although scientists have also shown that XIST can permit the expression of both alleles of TLR7 and other X-linked genes in some scenarios. The TLR7 gene, a 2018 study found, can escape silencing by X chromosome inactivation in immune cells from women and from men with Klinefelter syndrome who have additional X chromosomes.4 XIST can escape X inactivation as well, as has been documented in several cell types and disease states, although the exact mechanism responsible for the loss of XIST-mediated X inactivation remains unclear.5
In the new study, the team at Johns Hopkins conducted multiple searches for female sex-biased, self-RNA transcripts that can bind TLR7, based in part on recent studies showing that TLR7 has a high affinity for UU-containing RNA sequences. The assays converged on the 19 kb-long XIST transcript as the strongest candidate in the human genome. The researchers found it has the most pronounced female expression bias of any RNA transcript in adults: It was 472-fold more abundant in blood samples from female donors than from their male counterparts. The long noncoding RNA, the study found, is also a uniquely rich source of putative TLR7 binding sites, with an especially high binding capacity in its so-called “A-repeat region.”
A synthesized version of the XIST A-repeat region stimulated interferon production by human plasmacytoid dendritic cells expressing the TLR7 receptor. That interferon production, in turn, was wholly dependent on the presence of TLR7.
“A big unknown question when we sought to do this study was, ‘What are the specific self-RNAs that are actually driving—stimulating—toll-like receptor 7 in the setting of lupus, or is it any old cellular RNA?’” Dr. Darrah says. In female-derived epithelial cells, the researchers found, knocking out XIST expression significantly diminished the ability of the full pool of cellular RNAs to activate TLR7.
The experiment, in effect, allowed the researchers to ask how much XIST was contributing to the pool of potential TLR7 agonists. “The answer was a lot, because by completely deleting XIST, we lost a huge proportion of that signal downstream of TLR7,” Dr. Antiochos says. Out of the thousands of RNA transcripts, in fact, the loss of XIST reduced the signaling by 67%. “It really shows that there is some sequence specificity to this effect,” Dr. Darrah adds.
Given the previously suggested role of XIST in restraining TLR7 and protecting against lupus, “It was not intuitive that we would find, at the bulk level, more of it around in patients who have this condition,” Dr. Antiochos says. Nonetheless, the researchers found elevated levels of XIST in the blood leukocytes of women with SLE, compared to healthy controls. Those levels correlated positively with disease activity and the interferon signature: on average, women with clinical signs of disease had higher XIST levels than those with inactive disease. Likewise, the XIST levels correlated positively with the degree of disease severity as measured by Systemic Lupus Erythematosus Disease Activity Index scores.
“We saw this relationship between interferon and XIST, and one potential explanation of that would be that XIST itself could be induced by interferon,” Dr. Antiochos says. The team’s cellular assays had suggested that XIST could bind to TLR7 to induce type I interferon. But was a reverse mechanism, in which interferon could upregulate XIST through some kind of feedback loop, also possible?
To clarify the relationship, the researchers added interferon to a variety of cell types relevant for SLE pathogenesis and measured XIST levels before and after each treatment. The experiments showed that XIST expression levels didn’t change with the addition of interferon, meaning that XIST upregulation is likely not a downstream consequence of higher interferon levels in SLE. Instead, the results suggest a one-way relationship: Increased XIST expression is more likely the upstream driver of interferon upregulation.
Dr. Antiochos and Dr. Darrah suspect that dysregulation causing an overabundance of both the XIST ligand and TLR7 receptor may be necessary for the development of SLE. “That’s exactly the sort of situation you’d need for a disease that can perpetuate itself indefinitely,” says Dr. Antiochos. “You’d expect that one minor perturbation in the system wouldn’t be enough to generate the systemic disease that can go on for years. You’d have to have pathways that can really feed forward.”
As a disease trigger, the researchers think, extracellular vesicles released during cell apoptosis could provide a key source of extracellular self-RNA, including XIST. In live human cells undergoing apoptosis, they found that programmed cell death induced the trafficking of XIST transcripts to extracellular vesicles. That selective enrichment of XIST supports a model in which the self-RNA acts as a female-specific danger signal: Its packaging into the extracellular vesicles upon cell death stimulates TLR7-dependent secretion of interferon by plasmacytoid dendritic cells, thereby contributing to SLE development and increased disease activity.
An Excess of XIST
Montserrat Anguera, PhD, associate professor of biomedical sciences, University of Pennsylvania School of Veterinary Medicine, Philadelphia, says the study has helped unify several poorly understood observations in the field. “I really, really liked this paper,” says Dr. Anguera, who studies how X inactivation helps maintain the immune system but wasn’t involved with the new research.
On its own, the increased expression of XIST in the lymphocytes of patients with lupus may not seem especially noteworthy, she says. But the study helped clarify a functional reason for why that excess matters, “and that’s what we, in my lab, found so exciting about this study.”
Although XIST is normally associated with X inactivation via physical binding to one X chromosome, it and certain other transcripts can partially escape that inactivation. In T cells and B cells from patients with lupus, Dr. Anguera and colleagues have shown that the mechanism tethering XIST RNA to the X chromosome is impaired, leading to further escape.
“Not only are these cells transcribing more XIST, but they’re not able to keep the RNA locked down onto the chromosome,” she says. “So when you have a cell exploding and you have the release of the debris, you’re more likely to have these free RNA ligands able to bind to and then serve as a source for TLR7 activation.” What, specifically, is driving the tethering defect is unclear, but Dr. Anguera’s work suggests a clear association with lupus activity that can, in effect, increase TLR7 activation.
Dr. Anguera says the research also provides a new opening for investigating the relatively unexplored potential of hormone-mediated regulation on the X chromosome to help different regions escape silencing and spur the development of autoimmune diseases, such as lupus. “I get really excited thinking about how these two areas are going to come and interact,” she says.
The immunological phenomena, Dr. Antiochos notes, could be relevant for multiple diseases with a clear female bias, like Sjögren’s disease. For SLE, he says, higher levels of XIST RNA may help stratify the risk of disease development or future flares among women, although testing that potential will require longitudinal studies. The findings have raised the additional possibility of targeting the TLR7-XIST interaction as a therapeutic strategy. Because patients with SLE who have upregulated XIST would be expected to be more prone to exaggerated signaling through that pathway, Dr. Antiochos says, they may benefit from TLR7 inhibitors as well.
Kathrin Plath, PhD, a professor of biological chemistry, the University of California, Los Angeles, who has studied the role of XIST in initiating and maintaining X chromosome inactivation, says she’s intrigued by the new study. She cautions, however, that more in vivo data will be needed to confirm the role of XIST fragments as important TLR7 ligands. “Overall, [this is] an exciting avenue and it would be amazing if XIST enables female development, but then also causes autoimmunity in females,” Dr. Plath says.
Separate studies are providing independent support for the emerging hypothesis. One recent study in a mouse model of lupus showed that male mice engineered to produce XIST churned out autoantibodies and developed a more severe multi-organ pathology than mice lacking XIST. The finding, the authors report, suggests that complexes formed by XIST and bound proteins act as “antigenic triggers underlying the greater prevalence of autoimmune diseases in females.”6
As part of his own lab’s next steps, Dr. Antiochos hopes to understand more about how XIST travels from its initial attachment site on an X chromosome within the cell nucleus to extracellular vesicles in a way that activates TLR7.
“The other big question in my mind that is a direct result of this work is ‘Why is XIST up-regulated in these patients with lupus compared to healthy women?’” Dr. Antiochos says. If interferon doesn’t impact XIST expression in adults, what does? “That’s an area where there’s not a lot that’s understood,” he says.
Bryn Nelson, PhD, is a medical journalist based in Seattle.
References
- Brown GJ, Cañete PF, Wang H, et al. TLR7 gain-of-function genetic variation causes human lupus. Nature. 2022 May; 605(7909):349–356.
- Weckerle, CE and Niewold, TM. The unexplained female predominance of systemic lupus erythematosus: clues from genetic and cytokine studies. Clin Rev Allergy Immmunol. 2011 Feb; 40(1):42–49.
- Crawford JD, Wang H, Trejo-Zambrano D, et al. The XIST lncRNA is a sex-specific reservoir of TLR7 ligands in SLE. JCI Insight. 2023 Oct 23; 8(20):e169344.
- Souyris M, Cenac C, Azar P, et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018 Jan 26;3(19):eaap8855.
- Yu B, Qi Y, Li R, et al. B cell-specific XIST complex enforces X-inactivation and restrains atypical B cells. Cell. 2021 Apr 1;184(7):1790–1803.e17.
- Dou DR, Zhao Y, Belk JA, et al. Xist ribonucleoproteins promote female sex-biased autoimmunity. Cell. 2024 Feb 1;187(3):733–749.