Arnon Thongkonghan/shutterstock.com
Both due to its efficacy and favorable side effect profile when compared with alternative drugs for rheumatologic conditions, hydroxychloroquine is an important agent in rheumatologists’ armamentarium. However, one barrier to hydroxychloroquine use can be its effects on the eye (also see “Revised Retinopathy Screening Guidelines,”). Ocular side effects of hydroxychloroquine can include impact on the cornea, ciliary body, lens and retina.1 Corneal deposits, fortunately, rarely affect vison.2 However, the concern of retinal toxicity and the need for monitoring must be reviewed with patients prior to and during its use.2,3
Monitoring is intended to identify separate ocular disease that would interfere with identifying changes or baseline retinal disease that could increase hydroxychloroquine’s risk, as well as to detect the earliest changes related to hydroxychloroquine. The concern with retinal toxicity is that this may be irreversible and may progress even after hydroxychloroquine discontinuation. Identifying toxicity prior to changes affecting the fundus limits potential for further deterioration.4 Despite this, a significant proportion of individuals receive hydroxychloroquine without eye examinations.5
Prevalence
Estimates of prevalence are variable among studies, with differences likely representing the increasing availability of more sensitive testing and case ascertainment by the studies. Further, the impairment experienced by patients was variable. Initial studies demonstrated the risk as low as 0.5% and no events of retinal toxicity when dosing was below 6.5 mg/kg/day of ideal body weight.6 Further a prospective cohort demonstrated incidence of 0.5% in patients all treated with less than 6.5 mg/kg/day with no events occurring until after six years of treatment.7
Wolfe et al, in a study of patient-reported toxicity with review of medical records by experts, identified overall prevalence as 0.65%. However, the probability of toxicity was 0.29% after five years and further increased after 10 years (1%) and 15 years (2.1%).8 In a study published in 2014 by Melles and Marmor that included only patients who had been assessed with visual field examination or Spectral Domain-Optical Coherence Tomography (SD-OCT), the prevalence was 7.5%.9
Fortunately, thus far, studies have not identified clear risk to children who had an in utero exposure.10 In certain scenarios particularly systemic lupus erythematosus, hydroxychloroquine is felt to be an important treatment option for pregnant women.11
Ideal Body Weight Vs. Real Body Weight
In terms of daily dose, initial studies emphasized a cut-off of 6.5 mg/kg of ideal body weight in terms of toxicity risk.12 However, due to the typical dosing of either 200 or 400 mg of hydroxychloroquine, it is rare for individuals to receive higher than this cut-off. More recent studies suggest that real body weight predicts retinal toxicity better than ideal body weight.9 Further, a cut-off of 5 mg/kg/day based on real body weight has been proposed.
In the study by Melles and Marmor, doses higher than 5 mg/kg/day had rates of retinal toxicity of 10% within 10 years, which increased further after 20 years to 40%. In contrast, between 4 and 5 mg/kg, the risk was as low as 2% within 10 years and 20% after 20 years.9 This has been reflected in the most recent American Academy of Ophthalmology (AAO) retinal toxicity monitoring recommendations.13 Utilizing real body weight, typically up to a maximum of 400 mg, also has the advantage of being more convenient in the clinic.
Risk Factors
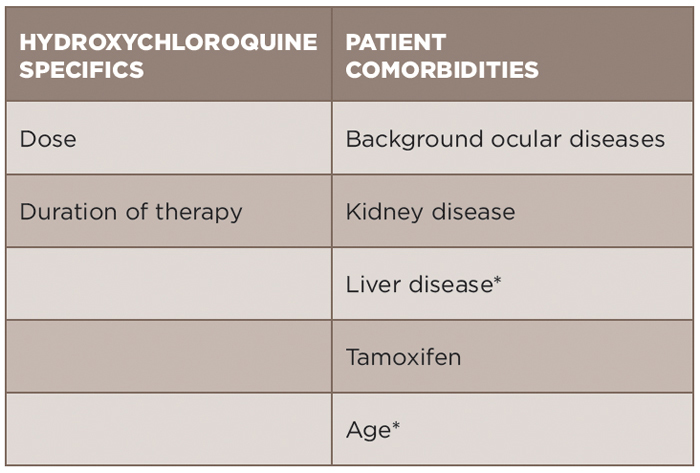
An individualized approach for assessment of risk of toxicity must be utilized (see Table 1). Age must be considered, in part because ocular diseases that accumulate with age could pose difficulty in terms of monitoring. Hydroxychloroquine is metabolized by the kidney and liver. Kidney disease has been demonstrated to increase the risk of toxicity (odds ratio 2.08) in the most recent study by Melles and Marmor.9 Liver disease in this same study was not identified to be associated with risk, but must be considered due its role in metabolism. Concurrent tamoxifen use has also been identified as a significant risk factor for retinal toxicity (odds ratio 4.59).9 However, blood level monitoring of hydroxychloroquine thus far has not been able to be used in the clinical setting to predict risk for retinal toxicity.2
Genetic factors are also being increasingly evaluated for their association with risk.14 Clinical trials are currently in process to further evaluate the role of genetics and hydroxychloroquine toxicity.
Screening
The American Academy of Ophthalmology (AAO) published updated retinal toxicity monitoring for hydroxychloroquine/chloroquine recommendations in 2016 (see Table 2).13 The dreaded consequence of hydroxychloroquine is “bull’s eye retinopathy,” which results in a ring scotoma. Ideally, earlier changes can be identified to prevent progression to this stage, which emphasizes the importance of screening.
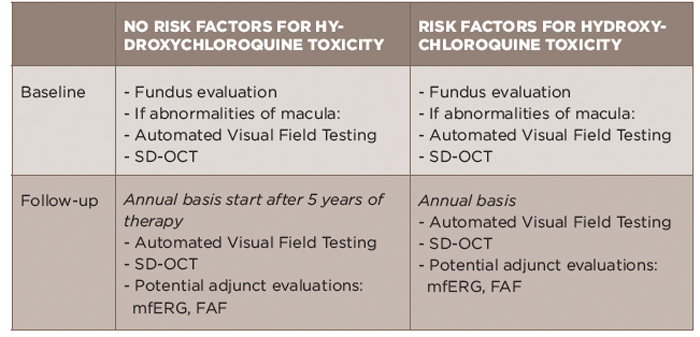
TABLE 2: Summary of 2016 AAO Screening Recommendations for Hydroxychloroquine Toxicity13
AAO: American Academy of Ophthalmology; FAF: fundus autofluorescence; mfERG: multifocal electroretinogram; SD-OCT: spectral domain-optical coherence tomography
The emphasis of baseline testing is the fundus evaluation of the macula. If abnormalities of the macula are noted, AAO would recommend proceeding with baseline visual fields and SD-OCT.13 The visual field testing should take into account a patient’s race/ethnicity as patients with Asian background can have toxicity that is not limited to the macula.15,16
For follow-up, automated visual fields and SD-OCT testing are recommended. The use of additional testing, including multifocal electroretinogram (mfERG) or fundus autofluorescence (FAF), is recommended when available at a patients’ eye care provider.13 SD-OCT generates images of the retina to the resolution level of cell layers. mfERG measures the electrical response to stimuli in order to identify damage. Fundus autofluorescence technology detects fluorescence that can be the consequence of damage.
Older forms of screening, including fundus photography, time-domain OCT, fluorescein angiography, full-field ERG, Amsler grid, color testing and electro-oculogram are not preferred because they are less sensitive and/or too subjective.
The first examination should occur at baseline (near, but not necessarily be prior to beginning therapy) and then, if no other risk factors are present, should be repeated after five years and annually thereafter. If additional risk factors are identified, then screening should occur annually.14
Although this specifically refers to screening in the asymptomatic setting, if a patient experiences vision changes including color vision then the patient should be referred to their eye care provider.
Managing Retinal Toxicity
The presence of damage necessitates discussion of discontinuation of hydroxychloroquine because there is no treatment. The patient, rheumatologist and ophthalmologist must collaborate to make the best decision for the individual patient. Equivocal results of testing can be followed up with repeat testing.2
Conclusion
Hydroxychloroquine can be an incredibly efficacious medication. Fortunately, retinal toxicity is uncommon, but assessment of risk and ophthalmic monitoring with objective tests is essential to reduce the impact. Rheumatologists must communicate with their patients to assure that monitoring is taking place. Risk factors should be taken into account when assessing the timing for surveillance including more recently identified concurrent tamoxifen use. Real body weight dosing is a more convenient option in the clinic, and doses less than 5 mg/kg/day have been demonstrated to correlate with lower risk.
The ACR has approved an updated position statement on screening for hydroxychloroquine retinopathy to emphasize the importance of screening and incorporation of a patient’s risk factors into screening.
Megan L. Krause, MD, is an assistant professor in the Division of Allergy, Immunology & Rheumatology, University of Kansas, Kansas City, Kans.
Vinicius Domingues, MD, is a clinical fellow in Rheumatology, Division of Rheumatology, New York University, New York.
Donald Miller, PharmD, is a professor of pharmacy practice at North Dakota State University, Fargo, N.D.
References
- Yam JC, Kwok AK. Ocular toxicity of hydroxychloroquine. Hong Kong Med J. 2006 Aug;12(4):294–304.
- Costedoat-Chalumeau N, Dunogue B, Leroux G, et al. et al. A critical review of the effects of hydroxychloroquine and chloroquine on the eye. Clin Rev Allergy Immunol. 2015 Dec;49(3):317–326.
- Ding HJ, Denniston AK, Rao VK, Gordon C. Hydroxychloroquine-related retinal toxicity. Rheumatology (Oxford). 2016 Jun;55(6):957–967.
- Marmor MF, Hu J. Effect of disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol. 2014 Sep;132(9):1105–1112.
- Nika M, Blachley TS, Edwards P, et al. Regular examinations for toxic maculopathy in long-term chloroquine or hydroxychloroquine users. JAMA Ophthalmol. 2014 Oct;132(10):1199–1208.
- Levy GD, Munz SJ, Paschal J, et al. Incidence of hydroxychloroquine retinopathy in 1,207 patients in a large multicenter outpatient practice. Arthritis Rheum. 1997 Aug;40(8):1482–1486.
- Mavrikakis I, Sfikakis PP, Mavrikakis E, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: A reappraisal. Ophthalmology. 2003 Jul;110(7):1321–1326.
- Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010 Jun;62(6):775–784.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014 Dec;132(12):1453–1460.
- Osadchy A, Ratnapalan T, Koren G. Ocular toxicity in children exposed in utero to antimalarial drugs: Review of the literature. J Rheumatol. 2011 Dec;38(12):2504–2508.
- Clowse ME, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 2006 Nov;54(11):3640–3647.
- Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: A report by the American Academy of Ophthalmology. Ophthalmology. 2002 Jul;109(7):1377–1382.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 Revision). Ophthalmology. 2016 Jun;123(6):1386–1394.
- Shroyer NF, Lewis RA, Lupski JR. Analysis of the ABCR (ABCA4) gene in 4-aminoquinoline retinopathy: Is retinal toxicity by chloroquine and hydroxychloroquine related to Stargardt disease? Am J Ophthalmol. 2001 Jun;131(6):761–766.
- Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015 Jan;122(1):110–116.
- Lee DH, Melles RB, Joe SG, et al. Pericentral hydroxychloroquine retinopathy in Korean patients. Ophthalmology. 2015 Jun;122(6):1252–1256.