Richard Quinn is a freelance writer in New Jersey.
References
ADVERTISEMENT
SCROLL TO CONTINUE
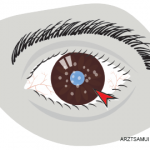
- AbbVie Inc. News release: AbbVie’s HUMIRA (adalimumab) receives U.S. Food and Drug Administration approval to treat adults with non-infectious intermediate, posterior and panuveitis. 2016 Jun 30.
- AbbVie Inc. News release: AbbVie receives orphan drug designation for Humira (adalimumab) from the U.S. Food and Drug Administration for the investigational treatment of certain forms of non-infectious uveitis. 2014 May 20.