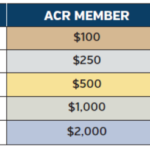
You are a rheumatologist in Texas. You are very well trained. Your mentors included some of the leaders in rheumatology, and you are respected by your colleagues and your patients. You know the devastation of untreated rheumatoid arthritis and lupus. A young woman with recent onset of systemic lupus erythematosus is your new patient. You…