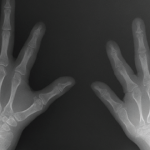
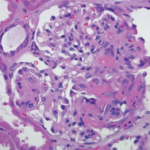
Rheumatologists and orthopedic surgeons must frequently collaborate to provide optimal patient care. Sometimes, they may even work at the same practice and form a care team for easy collaboration. Still, patient management from both specialties can be challenging, and specialists from both sides can learn from each other. How Crossover Starts Rheumatologists and orthopedic surgeons…