A 43-year-old woman presented to a rheumatologist for evaluation with a six-week history of arthralgias in the wrist, metacarpophalangeal (MCP), and proximal interphalangeal (PIP) joints. On examination, MCP joints were tender to palpation, and the left wrist and the right third and fourth PIPs were swollen. Because of a positive rheumatoid factor and anticyclic citrullinated peptide antibody test, the patient was diagnosed with rheumatoid arthritis (RA) and started on methotrexate and, subsequently, a tumor necrosis factor blocker. Although the inflammatory markers decreased and swelling subsided, the patient continued to have pain. In addition, she reported fatigue and inability to sleep. She also noted feeling sad and discouraged because pain limited the activities she could do. At this juncture, the rheumatologist was confronted with an important decision: to increase disease-modifying antirheumatic drug (DMARD) therapy or to develop an alternative plan to treat this patient’s pain. The key issue in the case focused on the origin of the pain. Was the pain from inflammation, or was it from something else?
Pain in Rheumatology
This scenario occurs almost every day in the offices of rheumatologists. Pain is the number one reason patients see a rheumatologist, and it is their highest priority when returning for follow-up. In a study of 2,795 patients who reported a physician diagnosis of RA, 86% stated that their disease was “somewhat to completely controlled,” but 64% were not satisfied with their pain levels. Although all of these patients were seeing a rheumatologist, 80% continued to rate their pain as “moderate to severe.”1
When faced with patients who have persistent pain despite seemingly adequate DMARD therapy, rheumatologists are often uncertain about the cause of pain and the best treatment strategy. According to the 2010 Report of the ACR Pain Management Task Force, rheumatologists “do not characterize themselves as ‘pain physicians’… Rheumatologists have traditionally approached pain from the perspective of the proximal causes of pain such as tissue injury and inflammation, and have concentrated therapy on reducing inflammation, either locally or systemically.”2
CNS Pain Mechanisms: Lessons from Fibromyalgia
This emphasis on inflammation as a therapeutic target to reduce pain is beginning to change, however, as neuroscience research reveals that, mechanistically, there are different types of pain. “Nociceptive” pain is generally considered adaptive and results from the appropriate detection of noxious stimuli (e.g., tissue injury or inflammation). “Pathological” pain is the product of damage to the central nervous system (CNS; e.g., neuropathic pain) or amplification of sensory signals in the CNS.3 Mounting evidence suggests that sensory amplification of pain and other stimuli may best explain the symptoms in syndromes like fibromyalgia.
To understand pain mechanisms that may operate in RA, fibromyalgia provides an important model. Fibromyalgia is a condition associated with chronic widespread pain. Many patients with this condition also experience fatigue, cognitive difficulties, sleep problems, and mood and anxiety disorders. In contrast to RA, fibromyalgia is not associated with obvious peripheral causes of pain; instead, studies have suggested that the etiology of fibromyalgia pain lies in the CNS.
The clinical pain experienced by fibromyalgia patients may be due to enhanced pain sensitivity. When exposed to noxious experimental stimuli, fibromyalgia patients report pain at lower stimuli intensities compared to controls.4 This increased pain sensitivity occurs at numerous sites across the body. Functional magnetic resonance imaging (fMRI) studies show corresponding changes in brain activity when fibromyalgia patients are exposed to a painful stimulus. These activations are not observed among healthy individuals who are exposed to a stimulus of the same magnitude but who do not consider the stimulus to be painful. However, if the magnitude of the stimulus is increased such that healthy individuals rate the stimulus at the same pain level as the stimulus provided to fibromyalgia patients, then brain activation is observed in the same areas.5 These observations strongly support the hypothesis that fibromyalgia patients are more sensitive to noxious stimuli than are healthy controls.
The CNS mechanisms that modulate pain in fibromyalgia involve: 1) dampening of descending analgesic pathways; and 2) augmentation of excitatory pain pathways (see Figure 1). The descending analgesic pathways originate in the cortical structures, hypothalamus, and brainstem and pass through the spinal cord to regulate sensory input from primary afferent fibers. These pathways act through serotonin, norepinephrine, and endogenous opioids, molecules that block excitatory neurotransmitter release, thereby dampening pain sensitivity. The descending serotonergic–adrenergic pathways are often impaired in fibromyalgia patients relative to those in healthy controls, and this impairment is associated with greater pain sensitivity and clinical pain severity. This deficit in inhibitory pain regulation is termed loss of “descending analgesia” or “diffuse noxious inhibitory controls.”
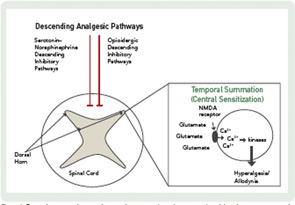
In addition to a loss of descending analgesia, some fibromyalgia patients also exhibit enhanced temporal summation, otherwise known as central sensitization. Temporal summation describes the phenomenon by which repetitive painful stimuli lead to the lowering of pain thresholds and heightened pain sensitivity. This process is mediated by the excitatory neurotransmitters, glutamate and substance P. These molecules activate N-methyl-D-aspartate (NMDA) receptor channels, leading to an influx of calcium ions which activate kinases that lower the pain threshold. These changes in CNS processing have implications for pharmacological and nonpharmacological treatment.
Pain Mechanisms in RA
In contrast to the situation in fibromyalgia, the role of augmented CNS pain processing in systemic inflammatory diseases, such as RA, is not well established. Several studies have reported that the prevalence of fibromyalgia is higher among patients with rheumatic diseases associated with peripheral pain and inflammation than among control populations. The prevalence of fibromyalgia ranges from 13% to 22% among patients with RA, compared to only 2.5% for healthy controls.6,7 Which RA patients are at risk for secondary fibromyalgia is not clear, although a recent study suggested that the development of fibromyalgia in RA is associated with psychosocial distress, comorbidities, and sociodemographic disadvantages.8
Several quantitative sensory studies have shown that RA patients are more likely to have widespread pain sensitivity than are healthy controls.9 This sensitivity occurs at both joint and nonjoint sites, suggesting a dysfunction in CNS pain processing rather than site-specific, peripheral changes. Although pain thresholds at joint sites are associated with both inflammatory (e.g., increases in C-reactive protein) and noninflammatory factors (e.g., sleep problems), pain thresholds at nonjoint sites are only associated with noninflammatory factors.10 This pattern of association is consistent with two mechanisms of pain: 1) a peripheral mechanism associated with inflammation; and 2) a central mechanism associated with a symptom complex that includes sleep problems, fatigue, and changes in mood.
To date, only one study has specifically assessed the role of descending analgesia in RA. This study documented a smaller magnitude of descending analgesia among RA patients compared to healthy controls, but the difference was not statistically significant.11
Pain Assessment in RA
Although future studies are needed to clarify the role of central pain mechanisms in RA, it is essential that rheumatologists implement comprehensive pain assessment and management strategies now. Routine pain assessment should include a thorough history and physical exam, as well as careful consideration of radiographic imaging. Key points to consider are the following: 1) the location of pain; 2) the presence of inflammation; and 3) the presence of structural damage (see Figure 2).
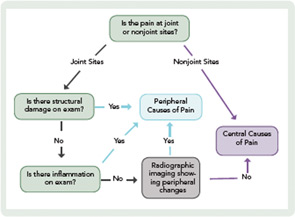
When assessing the location of pain, the main distinction is whether the pain is at joint or nonjoint sites. Pain at joint sites is more likely to be RA-related than pain at nonjoint sites. However, many patients may have pain at joint sites but no signs of inflammation or structural damage on physical examination. In these patients, it may be helpful to perform radiographic imaging via ultrasound or MRI to look for evidence of synovitis or erosions.
In the absence of radiographic findings, dysregulation of central pain processing mechanisms should be suspected, prompting the assessment of comorbid fibromyalgia. This assessment may be aided by the new ACR clinical and survey criteria for the diagnosis of fibromyalgia.12,13 These criteria do not require physical examination but are based on self-report measures that assess widespread pain and other symptoms (see Table 1).13 It is also important to ask the patient about fatigue, poor sleep, subjective cognitive problems, and/or psychiatric comorbidity (predominantly mood disturbance and anxiety). These symptoms are frequently present and can contribute, either individually or in concert, to the pain experience.
Pain Management in RA
The treatment of pain in RA should be multifaceted. Although it is essential to control inflammation using immunomodulatory agents, it is also important to treat pain and promote functioning. To accomplish this goal, comprehensive management strategies should include nonpharmacologic methods, such as self-management programs that are commonly used to treat other chronic pain conditions such as fibromyalgia.14 In addition, clinicians also may consider pharmacologic treatments to address central pain processing deficits.
Self-management programs are geared toward educating patients about their conditions, teaching coping skills, improving functional status, and preventing relapses. The Arthritis Foundation Self-Help Program is a six-week group course that teaches methods to manage pain, fatigue, and stress. Several studies have shown short-term improvements in pain among patients who complete self-management programs.15
The data supporting the short- and long-term effectiveness of cognitive–behavioral therapy approaches are quite good. Patients with arthritis are taught skills, including activity pacing, goal setting, problem solving, relaxation, imagery, and cognitive restructuring to improve pain control.16 Web-based tools, like the Psychologist Locator on the website of the American Psychological Association (http://locator.apa.org), facilitate finding a practitioner in the community. In addition, efforts are ongoing to make these interventions more accessible through programs provided in the clinic by allied health professionals or accessible online directly by patients.
Solid evidence also exists for the integration of exercise into pain management programs for individuals with RA.17 Controlled trials in RA have evaluated aerobic training, strength training, and a combination of the two. Overall, these studies reported good outcomes, including decreased pain and improvements in physical functioning, muscle strength, aerobic capacity, disease activity, mood, and fatigue. As is the case with most interventions, patients with RA undertaking a new exercise regimen should be carefully evaluated beforehand, and the program tailored to the patient’s needs and abilities. Low-impact land- or pool-based exercise of low to moderate intensity engaged in two to three times a week is thought to be helpful for fibromyalgia pain and may generalize to persistent pain in RA.18 Local YMCAs often offer aqua aerobics classes, and organized mall-walking groups abound. Community chapters of the Arthritis Foundation can be helpful to locate appropriate regional exercise resources.
Pharmacologic therapies may include medications typically used to treat chronic widespread pain syndromes. The most data exist regarding the efficacy of tricyclic antidepressants in RA. Tricyclic antidepressants inhibit serotonin and norepinephrine reuptake, which may affect central pain processing through the inhibitory descending serotonin–norepinephrine pathways. Some small studies have reported reductions in pain among RA patients taking tricyclic antidepressants compared with those treated with placebo.19
It is not known whether Food and Drug Administration (FDA)–approved medications for fibromyalgia, such as duloxetine, milnacipran, and pregabalin, are effective in treating pain in RA. Duloxetine and milnacipran are serotonin–norepinephrine reuptake inhibitors and likely work through the inhibitory descending serotonin–norepinephrine pathways. Pregabalin binds to calcium channels, preventing the release of neurotransmitters, including serotonin, norepinephrine, and glutamate. These medications are effective in a variety of other musculoskeletal pain conditions, and duloxetine recently received FDA approval for the treatment of chronic pain due to osteoarthritis and low back pain. If RA patients have a component of central pain, these medications should theoretically be efficacious adjunctive treatments for pain. However, most studies of these medications have specifically excluded patients with systemic inflammatory diseases including RA.
Future Directions
Although rheumatologists have become adept at controlling inflammation with disease-modifying drugs and biologic agents, pain continues to plague a substantial proportion of our patients. The next decade will be an important time for the development of pain research in rheumatology. The ACR has recognized the need for more education and research involving pain and pain management, and the report of the ACR Pain Management Task Force shows just how far we still have to go.20 It is time for clinicians and researchers to acknowledge pain as both a clinical and research priority. Pain in RA should no longer be lost in the shadow of inflammation but rather be seen as an important entity in and of itself.
Dr. Lee is an instructor in medicine, Division of Rheumatology, Immunology, and Allergy, Brigham and Women’s Hospital, in Boston. Dr. Hassett is an associate research scientist, Department of Anesthesiology, University of Michigan Medical School, Chronic Pain and Fatigue Research Center, in Ann Arbor, Mich.
References
- Taylor P, Manger B, Alvaro-Gracia J, et al. Patient perceptions concerning pain management in the treatment of rheumatoid arthritis. J Int Med Res. 2010;38:1213-1224.
- Borenstein D, Altman R, Bello A, et al. Report of the American College of Rheumatology Pain Management Task Force. Arthritis Care Res. 2010;62:590-599.
- Woolf CJ. What is this thing called pain? J Clin Invest. 2010;120:3742-3744.
- Geisser ME, Casey KL, Brucksch CB, Ribbens CM, Appleton BB, Crofford LJ. Perception of noxious and innocuous heat stimulation among healthy women and women with fibromyalgia: Association with mood, somatic focus, and catastrophizing. Pain. 2003;102:243-250.
- Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333-1343.
- Ranzolin A, Brenol JC, Bredemeier M, et al. Association of concomitant fibromyalgia with worse disease activity score in 28 joints, health assessment questionnaire, and short form 36 scores in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61:794-800.
- Coster L, Kendall S, Gerdle B, Henriksson C, Henriksson KG, Bengtsson A. Chronic widespread musculoskeletal pain: A comparison of those who meet criteria for fibromyalgia and those who do not. Eur J Pain. 2008;12:600-610.
- Wolfe F, Hauser W, Hassett AL, Katz RS, Walitt BT. The development of fibromyalgia – I: Examination of rates and predictors in patients with rheumatoid arthritis (RA). Pain. 2011;152:291-299.
- Gerecz-Simon EM, Tunks ER, Heale JA, Kean WF, Buchanan WW. Measurement of pain threshold in patients with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and healthy controls. Clin Rheumatol. 1989;8:467-474.
- Lee YC, Chibnik LB, Lu B, et al. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: A cross-sectional study. Arthritis Res Ther. 2009;11:R160.
- Leffler AS, Kosek E, Lerndal T, Nordmark B, Hansson P. Somatosensory perception and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from rheumatoid arthritis. Eur J Pain. 2002;6:161-176.
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol. 2011;38:1113-1122.
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600-610.
- Hassett AL, Gevirtz RN. Nonpharmacologic treatment for fibromyalgia: Patient education, cognitive-behavioral therapy, relaxation techniques, and complementary and alternative medicine. Rheum Dis Clin North Am. 2009;35:393-407.
- Iversen MD, Hammond A, Betteridge N. Self-management of rheumatic diseases: State of the art and future perspectives. Ann Rheum Dis. 2010;69:955-963.
- Keefe FJ, Somers TJ. Psychological approaches to understanding and treating arthritis pain. Nature Rev. 2010;6:210-216.
- Cairns AP, McVeigh JG. A systematic review of the effects of dynamic exercise in rheumatoid arthritis. Rheumatol Int. 2009;30:147-158.
- Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79.
- Ash G, Dickens CM, Creed FH, Jayson MI, Tomenson B. The effects of dothiepin on subjects with rheumatoid arthritis and depression. Rheumatology (Oxford). 1999;38:959-967.
- Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13:211.


