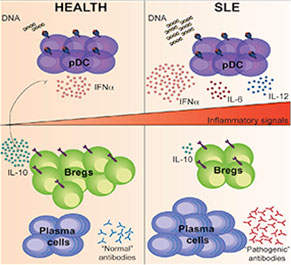
Graphical Abstract: The signals required for Breg cell differentiation in humans are currently unknown.
Regulatory B (Breg) cells appear to play a critical role in managing the immune system and maintaining homeostasis. In healthy individuals, Breg cells are generated in response to pro-inflammatory cytokines secreted, at least in part, by plasmacytoid dentricitic cells (pDCs). Thus, pDCs appear able to both initiate a pro-inflammatory response and trigger the differentiation of regulatory cells. Given this important interaction, it is not surprising that researchers have examined the role of both Breg cells and pDCs in inflammatory diseases. Although researchers have been closing in on the relationship between pDCs and Breg cells, until now, the signals required for Breg cell differentiation in humans have remained unknown.
Madhvi Menon, a graduate student at the University College London, and colleagues published their analysis of pDCs in the March 15 issue of Immunity.1 The authors began by investigating whether pDCs could, indeed, generate Breg cells. They found that, when compared with B cells stimulated with the toll-like receptor 9 (TLR9) agonist CpGC alone, stimulation with pDCs induced a four-fold enrichment of IL-10-producing B cells. Moreover, when the B cells were cultured with either CpGC or with pDCs plus CpGC, they found that B cells cultured with pDCs were able to suppress the differentiation of IFNɣ+CD4+ T cells and TNFα+CD4+ T cells. Thus, the investigators established that pDCs control the differentiation of immature B cells into CD24+CD38hi Breg cells or plasmablasts via the secretion of IFNα. The differentiated Breg cells were then able to use IL-10 to limit pDC production of IFNα.
“Next, we investigated whether the pDC-expanded CD24+CD38hi Breg cells were derived directly from immature B cells or whether pDCs upregulated the expression of CD24 and CD38 on mature and memory B cells,” explained the authors. They purified B cell subsets from the pDC-immature B cell coculture and then co-cultured the cells with anti-CD3 CD4+CD25- T cells.
“We found that the concentration of IFNα determines whether an immature B cell develops into a CD24+CD38hi Breg cell or a plasmablast,” write the authors in their discussion. “At lower concentrations of IFNα, B cells differentiate into both plasmablasts and CD24+CD38hi Breg cells, whereas at higher concentrations, the B cell response is channeled toward plasma cell maturation.”
When the investigators turned their attention to the CD24+CD38hi B cells in patients with systemic lupus erythematosus (SLE), they found that these patients had defective pDC-mediated expansion of CD24+CD38hi Breg cells. Specifically, patients with SLE had hyperactivated pDCs that failed to induce Breg cells. However, the exceptions to this observation were patients with SLE who responded to rituximab. This subset of patients displayed a normalized pDC-Breg cell interaction.