Osteoporosis is a serious, but preventable condition. Hip and vertebral fractures are associated with increased disability, mortality and prolonged care at nursing facilities. In 2005, it was estimated that the cost of osteoporosis-related fractures totaled $17 billion.1
Osteoporosis is more prevalent in aging populations, who often have multiple comorbidities. Patients with impaired renal function are especially challenging because they have a higher incidence of osteoporosis and metabolic bone disease associated with chronic kidney disease (CKD). This includes adynamic bone disease, uremic osteodystrophy, and abnormalities of parathyroid hormone (PTH), vitamin D, calcium and phosphorus. Treatment options are limited in patients with chronic kidney disease (CKD) because bisphosphonates are contraindicated when the creatinine clearance is below 35 mL/min.
Denosumab was approved by the Food and Drug Administration for treatment of osteoporosis in 2010 and is one of the few medications that has been used as a treatment option in patients with CKD.2 Denosumab is a human monoclonal antibody with affinity to receptor activator of nuclear factor kappa B ligand (RANKL). It prevents RANKL/RANK interaction, leading to inhibition of osteoclast formation, function and survival.2 We report the development of persistent and symptomatic hypocalcemia in an older patient who was treated with this drug.
We suggest caution when using denosumab in patients with severe renal impairment (GFR <30 mL/min).
Case
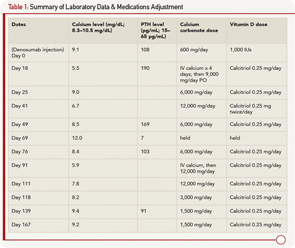
A 79-year-old man with a history of gout, rheumatoid arthritis treated with corticosteroids, type 2 diabetes mellitus and stage 4 CKD due to focal segmental glomerulosclerosis was evaluated at a rheumatology clinic for increased risk for future fracture based on low T scores of –1.9 at the lumbar spine and –1.7 at the femoral neck. The FRAX (Fracture Risk Assessment Tool) calculated 10-year risk for hip fracture was 5.5%. His creatinine was 2.7 mg/dL (normal 0.5–1.0 mg/dL) with an estimated GFR of 25 mL/min (normal >60 mL/min), calcium 9.1 mg/dL (normal 8.3–10.5), 25 (OH) vitamin D 25.0 mg/mL (normal 30–100), phosphorus 3.4 mg/dL (normal 2.5–4.8 mg/dL) and PTH 108 pg/mL (normal 15–65). Sixty mg of denosumab via subcutaneous injection was given to the patient as an approved option in patients with CKD. Prior to injection, the patient was taking 600 mg a day of calcium carbonate and 1,000 units/day of vitamin D3.
Two weeks later, the patient developed malaise and upper extremity weakness. Within several days, he was admitted to the hospital with fever of 39.6ºC, malaise, myalgia, tremor, irritability and paresthesias. He was found to have severe hypocalcemia with a calcium level of 5.5 mg/dL, ionized calcium of 0.90 mmol/L (1.13–1.32 mmol/L), PTH 190 and creatinine of 3.1 mg/dL. He was evaluated by rheumatology, endocrinology and nephrology, and was diagnosed with hypocalcemia due to denosumab. The patient was treated with IV calcium for four days and subsequently transitioned to oral calcium carbonate 2,250 mg four times a day, and calcitriol 0.25 mg daily. The patient remained in the hospital for eight days due to persistent hypocalcemia. At discharge, his serum calcium was 9.0, creatinine was 2.5 and he was discharged on calcium carbonate 1,500 mg four times a day and calcitriol 0.25 mg daily.