ustas7777777 / shutterstock.com
Recent data showing sustained, long-term safety and efficacy of mavrilimumab for the treatment of rheumatoid arthritis (RA) confirm and build on prior evidence from phase 2 studies of the potential for this new agent for the treatment of RA.
Mavrilimumab is a human monoclonal antibody that blocks a proinflammatory cytokine involved in RA pathogenesis—granulocyte-macrophage colony-stimulating factor (GM-CSF)—and is the first agent targeting the receptor of this cytokine.
The study found mavrilimumab remained well tolerated with only mild or moderate adverse events and no signs of pulmonary deterioration. In addition, long-term efficacy was maintained.1
“These results support data from previous studies,” says lead author of the study, Gerd R. Burmester, MD, professor of medicine and director of the Department of Rheumatology and Clinical Immunology, Charité-University Medicine Berlin, Germany.
Unique to this study was the use of multiple pulmonary function tests to provide a longitudinal, long-term assessment of pulmonary function. A particularly important finding was that no pulmonary deterioration occurred with long-term use of mavrilimumab. Identifying long-term effects of mavrilimumab on pulmonary function is particularly important given the potential harmful effects of blocking GM-CSF, which plays a critical role in lung homeostasis and promoting alveolar macrophage development.
A particularly important finding was that no pulmonary deterioration occurred with long-term use of mavrilimumab.
A Closer Look

Dr. Burmester
In the study, Dr. Burmester and his colleagues pulled together data from three prior studies of mavrilimumab to assess sustained safety and efficacy for up to three years.2-4 Table 1 lists the studies.
When combining the safety data from these studies, Burmester and colleagues found that 442 patients from these three studies received 100 mg mavrilimumab every other week with a cumulative mavrilimumab safety exposure of 899 patient-years. Of the treatment-emergent adverse events reported, most were mild to moderate, with nasopharyngitis as the most common (n=69; 7.68 per 100 patient-years) followed by bronchitis (n=51; 5.68 per 100 patient-years). The study also found low incidences of neutropenia (0.90%; 0.54 per 100 patient-years) and serious infections (3.17%; 1.56 per 100 patient-years). There were no reports of monocytopenia.
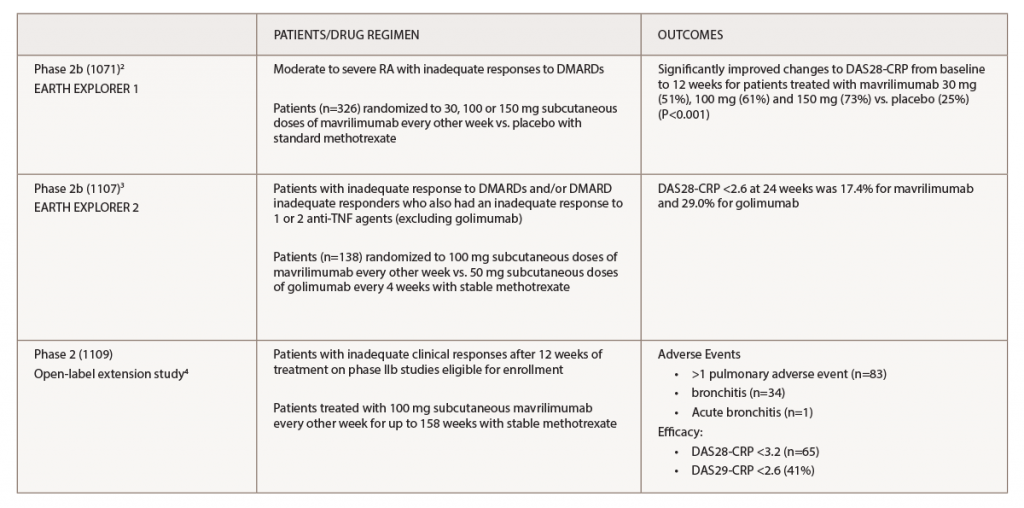
(click for larger image) Table 1. Key: DAS28-CRP, Disease Activity Score in 28 Joints; DMARDs, disease-modifying anti-rheumatic drugs; TNF, tumor necrosis factor
“Importantly,” says Dr. Burmester, “pulmonary deterioration was not evident with long-term mavrilimumab treatment in addition to standard care.”
Pulmonary function and long-term pulmonary safety are listed in Table 2. The multiple pulmonary tests used to test lung function and long-term safety included serial standardized lung function testing (FEV1/FVC), assessments of dyspnea using the Borg Dyspnea Index and chest radiographs.