 Physicians prescribe multiple medications, both on and off label, in an attempt to manage systemic lupus erythematosus (SLE). These medications include antimalarials, non-steroidal anti-inflammatory drugs, aspirin, corticosteroids, azathioprine, mycophenolate, cyclophosphamide, methotrexate, belimumab and rituximab. Unfortunately, because of their limited efficacy, these drugs are often prescribed in different combinations that may result in the added risk of toxicity. Thus, although patients with SLE do have multiple treatment options, SLE remains a disease with significant morbidity and mortality, and patients with poor control of their disease can progress to end-stage organ involvement.
Physicians prescribe multiple medications, both on and off label, in an attempt to manage systemic lupus erythematosus (SLE). These medications include antimalarials, non-steroidal anti-inflammatory drugs, aspirin, corticosteroids, azathioprine, mycophenolate, cyclophosphamide, methotrexate, belimumab and rituximab. Unfortunately, because of their limited efficacy, these drugs are often prescribed in different combinations that may result in the added risk of toxicity. Thus, although patients with SLE do have multiple treatment options, SLE remains a disease with significant morbidity and mortality, and patients with poor control of their disease can progress to end-stage organ involvement.
Therefore, SLE patients require new treatment options that can effectively reduce disease activity, disease flares and the requirement for corticosteroid and cytotoxic drugs. They also need a drug with an acceptable safety profile that can improve quality of life by controlling pain and fatigue and, ultimately, delay organ damage. Baricitinib, a Janus kinase (JAK) 1 and 2 inhibitor, is being examined to meet this need.
Research indicates that JAK and the signal transducer and activator of transcription (STAT) play a key role in the cytokine-mediated immune signaling and inflammation associated with SLE. This apparently critical pathway includes JAK/STAT-mediated signaling through type 1 interferons. Baricitinib is an oral selective JAK 1 and JAK 2 inhibitor that may be an effective treatment for SLE patients, according to promising results from a recent phase 2 trial.
The study revealed that, at 24 weeks 4 mg of baricitinib significantly improved the signs and symptoms of active SLE in patients who were not adequately controlled despite standard of care therapy. Daniel J. Wallace, MD, clinical professor of medicine at the David Geffen School of Medicine at the University of California, Los Angeles, and colleagues also found the 2 mg dose of baricitinib was not effective. These results, published July 21 in Lancet, in combination with similar positive results from additional studies, will provide the foundation for future phase 3 clinical trials evaluating baricitinib as a potential oral therapy for SLE.1
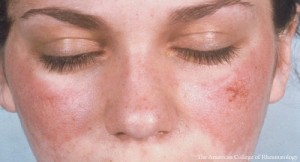
“We chose the primary objective, resolution of arthritis or rash by SLEDAI-2K (Systemic Lupus Erythematosus Disease Activity Index-2), for this phase 2 trial because arthritis and rash are among the most common manifestations of [SLE] and are the two most frequent clinical manifestations at entry in recent clinical trials of extra-renal systemic lupus erythematosus,” write the authors in their discussion. “The SLEDAI-2K organ domain scoring is a validated, widely used outcome measure for the study of [SLE]. Arthritis, as well as musculoskeletal pain, is a prominent symptom in patients with [SLE], and improvement in musculoskeletal symptoms, assessed with several measures, is associated with improvement in quality of life.”
At Week 24, researchers found 67% of patients receiving 4 mg baricitinib achieved resolution of SLEDAI-2K arthritis or rash. The safety profile of baricitinib in patients with active SLE receiving standard of care treatment was consistent with previously published findings. The 4 mg treatment group experienced a single episode of deep-vein thrombosis. Baricitinib treatment also resulted in modest dose-associated decreases in hemoglobin neutrophil and triglyceride concentrations, as well as increases in platelet counts, concentrations of creatine phosphokinase, HDL cholesterol and total cholesterol.
Lara C. Pullen, PhD, is a medical writer based in the Chicago area.
Reference
- Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2018 Jul 21;392(10143):222–231. doi: 10.1016/S0140-6736(18)31363-1.



