Over the last year, there has been increasing interest in other targets displayed in Figure 1 (above). For example, interleukin (IL) 4, which is increased in the bronchoalveolar lavage fluid, blood, and skin of patients with SSc might be considered as a possible target for therapy.29 Radstake et al point out the possible role of IL1, IL17, and Th17 cells in this disease.30 In addition, CTGF presents a possible target for systemic sclerosis.31 While definitive therapy is not yet at hand, the past year indicates the possibility that targeted therapy may assume a role in at least some aspects of SSc.
Stem Cell Transplantation
As described above, progress continues in our understanding of the biology and pathogenesis of SSc. Similarly, progress has been observed in the application of hematopoietic cell transplantation (HCT) for the treatment of severe autoimmune diseases. In the 1980s, animal experiments in genetic or antigen-induced models of autoimmune disorders demonstrated that following myeloablative conditioning, allogeneic or autologous HCT can prevent disease progression and even reverse organ damage in, respectively, inherited or acquired models of autoimmune disease.32,33
Remarkably, studies of post-transplant immune recovery showed that the reconstituted immune repertoire following lymphoablation and autologous HCT demonstrated repair of baseline immune defects and alterations in adoptive immune responses associated with the autoimmune disease.34 Following allogeneic HCT, a normal donor immune system is engrafted in the transplant recipient. With CD34+ selected autologous HCT, the immune network is normalized and “reset” via repertoire replacement.34 At present, studies are ongoing to determine whether and to what extent suppression of inflammation after HCT is due to re-regulation of immunity or abolition of populations of disease-associated T or B cells.
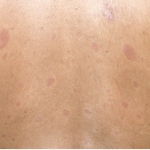
Completed clinical pilot studies provide data with sufficient follow-up to weigh the efficacy and safety of HCT for autoimmune diseases.35 More than 1,000 transplants for autoimmune diseases have been performed in Europe. Ongoing phase III multinational European trials are being conducted in SSc (the ASTIS trial), multiple sclerosis (the ASTIMS study), and Crohn’s disease (the ASTIC trial). Completed phase II trials in the United States of HCT for SSc have shown promising outcomes. Figures 2 (p. 21) and 3 (p. 22) demonstrate significant improvement/resolution of dermal sclerosis and stabilization/improvement in pulmonary function persisting beyond five years after autologous HCT.36 A follow-up study is comparing treatment with chronic high-dose pulse immunosuppression versus lymphoablation and autologous CD34+ selected autologous HCT. This phase III randomized study is currently recruiting patients in Canada and the United States who have severe SSc and internal organ involvement for enrollment in the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial. Most commonly, individuals with diffuse scleroderma are enrolled who have baseline FVC or DLCO values <70% predicted. Table 2 (p. 22) provides an overview of the eligibility and referral information with further details found at the study Web site (www.sclerodermatrial.org). Within the trial are imbedded a number of NIH-supported mechanistic studies to further understand the molecular mechanisms of SSc, immune regulation, and response to treatment.