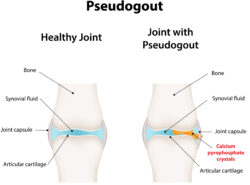
PHILADELPHIA—The term pseudogout has been a source of confusion among rheumatologists, especially as our understanding of calcium pyrophosphate crystal deposition (CPPD) disease has become more nuanced. This condition has many complex and interesting facets that warrant closer investigation.
An ACR Convergence 2022 session sought to update the audience on the progress to date in developing classification criteria for CPPD disease. The first speaker was Sara Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School and Brigham and Women’s Hospital, Boston. She discussed the methodology and item considerations in developing the new classification criteria.1
Dr. Tedeschi noted that CPPD disease is common, but research on the condition has been stymied by the lack of validated classification criteria, reliance on synovial fluid polarized light microscopy for diagnosis and the disease’s diversity of presentations. These presentations can include acute CPP crystal arthritis (i.e., the condition historically called pseudogout), chronic CPP crystal inflammatory arthritis, osteoarthritis with CPPD and crowned dens syndromes.
Developing Classification Criteria
Using a four-phase process that began in 2019, the group working on the classification criteria used data and expert opinion from rheumatologists, a musculoskeletal radiologist and a methodologist from across the country and world to advance the project.
The first phase of the process was item generation, which included a literature review and the solicitation of suggestions from patients and a combined expert committee. The second phase was item reduction and refinement, which included discussions with an advisory group focused on clinical, laboratory and imaging data, as well as an item rating exercise by combined expert committee members. Here, Dr. Tedeschi referenced a paper she wrote with colleagues in which imaging features characteristic of CPPD on radiographs, computed tomography (CT) scans and dual-energy CT (DECT) studies were defined and a set of example images was compiled.2
In the study’s third phase, item weighting and threshold scoring was pursued. The group assembled a derivation cohort, which refers to a group of patients who would be used for testing some of the features of the classification criteria framework. Rheumatologists from 13 sites and six countries provided data from de-identified patients and were asked to rate their clinical suspicion for CPPD disease on a scale of -3 (least likely) to +3 (most likely).
Next, two blinded expert adjudicators from different institutions reviewed each case. The group then used data from the adjudicators, the rheumatologists who submitted the cases and information from synovial fluid analysis (when present) to define three patient categories: definite cases, definite mimickers (who then served as the control group) and uncertain. The group identified features of clinical cases that increased the odds ratio (OR) of a patient’s likelihood of having CPPD disease, such as if the patient ever had an acute inflammatory arthritis of the knee (OR 3.0, 95%; confidence interval [CI] 1.9–4.8) or the patient having more than one typical episode of acute inflammatory arthritis (OR 4.1, 95%; CI 2.5–6.7).
In her discussion, Dr. Tedeschi described the entry criteria, absolute exclusion criteria and sufficient criteria for the CPPD disease classification criteria framework. Importantly, the entry criterion requires a patient to have had at least one episode of joint pain, swelling or tenderness, and the absolute exclusion criteria are if all symptoms are more likely explained by an alternative diagnosis, such as rheumatoid arthritis, gout, osteoarthritis, etc.
Dr. Tedeschi noted that it’s important to evaluate patients for related metabolic disease associated with CPPD, such as hereditary hemochromatosis, primary hyperparathyroidism, hypomagnesemia or hypophosphatasia.
To conclude her presentation, Dr. Tedeschi described the fourth phase of the process, which involved validation of the criteria using a recruited cohort of patients and entailed calculations of sensitivity and specificity.
Item Weighting
The session’s second speaker was Abhishek Abhishek, MBBS, MD, FRCP, PhD, professor of rheumatology, The University of Nottingham, U.K., who talked about item weighting in greater detail.
He noted that the combined expert committee members were presented with paired patient scenarios describing such features as sites of typical inflammatory arthritis and number of peripheral joints with imaging evidence of CPPD. The members were then asked to vote on which patient in the pair was more likely to have CPPD disease. A facilitator then encouraged discussion among committee members until a consensus was reached on each pair of patients.
The members identified two criteria, crowned dens syndrome and synovial fluid analysis demonstrating CPP crystals in a joint with swelling, tenderness or pain, as sufficient criteria for classification of CPPD disease.
Dr. Abhishek noted that calculations were made to identify a threshold score from the classification criteria that could be used to optimize sensitivity and specificity. By looking at the derivation cohort of more than 400 patients, with 190 definite cases of CPPD disease, 148 mimickers and 80 uncertain cases, a score of greater than or equal to 57 yielded a specificity of 87.9% and a sensitivity of 92.2%. This threshold score of 57 also performed well in the validation cohort.
Dr. Abhishek concluded by commenting on behalf of Hyon Choi, MD, DrPH, professor of medicine, Harvard Medical School, General Hospital, Boston. He noted that imaging domains account for nearly half of the weighting in the classification criteria and that sensitivity is higher for ultrasound, CT imaging and DECT studies than for conventional radiography.
Additionally, he pointed out that it can be difficult to distinguish between CPPD disease and basic calcium pyrophosphate deposition on imaging, but the imaging definitions from the study by Dr. Tedeschi and colleagues may help with this issue.2
Finally, Dr. Abhishek noted several practical limitations for using presence of CPPD crystals in synovial fluid as the gold standard for diagnosis. These issues include low sensitivity with a high false-negative rate, variability between observers (i.e., two individuals evaluating the same synovial fluid may not always concur as to the presence or absence of CPPD crystals), the crystals themselves can be hard to detect due to small size and absent or weak positive birefringence under polarized light, and obtaining synovial fluid is sometimes difficult, especially from small joints.
Thanks to Dr. Tedeschi, Dr. Abhishek and collaborators, the future of CPPD disease diagnosis and research looks as striking as a band of chondrocalcinosis on an X-ray.
Jason Liebowitz, MD, completed his fellowship in rheumatology at Johns Hopkins University, Baltimore, where he also earned his medical degree. He is currently in practice with Skylands Medical Group, N.J.
References
- Tedeschi SK, Pascart T, Latourte A, et al. Identifying potential classification criteria for calcium pyrophosphate deposition disease: Item generation and item reduction. Arthritis Care Res (Hoboken). 2022 Oct;74(10):1649–1658.
- Tedeschi SK, Becce F, Pascart T, et al. Imaging features of calcium pyrophosphate deposition (CPPD) disease: Consensus definitions from an international multidisciplinary working group. Arthritis Care Res (Hoboken). 2022 Apr 19. Online ahead of print.