PHILADELPHIA—Selecting my top 10 picks for abstracts in psoriatic arthritis (PsA) at the ACR Convergence 2022 meeting was not easy because there was a great deal to review and learn from the 139 abstracts submitted to the meeting. I focused first and foremost on advances in therapeutics that encompassed both new and approved therapeutics, novel developments in molecular imaging and new insights into the mechanism of pain hypersensitivity.
1. Abstract 1599
Bimekizumab Treatment in Patients with Active Psoriatic Arthritis and Inadequate Response to Tumor Necrosis Factor Inhibitors: 16-Week Efficacy and Safety from a Phase 3, Randomized, Double-Blind, Placebo-Controlled Study. Research by Merola J, et al.1
Bimekizumab is a bispecific monoclonal antibody that inhibits both interleukin (IL) 17A and IL-17F cytokines. Inhibitors of IL-17A are highly effective therapies approved for the treatment of both psoriasis and psoriatic arthritis (PsA). IL-17F enhances inflammation induced by IL-17A in in vitro and animal models relevant to PsA and clinical trials comparing bimekizumab with IL‑17A‑specific inhibition in psoriasis suggest that it may lead to more effective suppression of inflammation.
The phase 3 BE COMPLETE study assessed 160 mg of BKZ or placebo every four weeks in 400 patients with active PsA and inadequate response to a tumor necrosis factor inhibitor (TNFi). At the week 16 primary end point, there were significantly more ACR50 responders in the bimekizumab group (43.4%) than in the placebo group (6.8%). Just over half of patients on bimekizumab achieved complete skin clearance (PASI 100) and just under half achieved resolution of nail psoriasis and minimal disease activity (MDA). Treatment was well tolerated; the most frequent adverse events were mild to moderate oral candidiasis (bimekizumab: 2.6%, placebo: 0%) and nasopharyngitis (bimekizumab: 3.7%, placebo: 0.8%).
Bimekizumab adds to a growing list of therapeutics that are effective for both joint and skin disease in patients with PsA, but further trials are necessary to determine if dual inhibition of IL-17A and IL-17F offers advantages over agents that inhibit only IL-17A. Further data demonstrating efficacy for axial inflammation would also be helpful.
2. Abstract 1597
Achievement of Different Treatment Targets with Izokibep Demonstrates Efficacy Benefits in Patients with Active Psoriatic Arthritis: Results from a 16-Week Randomized, Placebo-Controlled Phase 2 Clinical Trial. Research by Behrens F, et al.2
Izokibep is a first-in-class fusion protein demonstrating high binding potency for IL-17A, binding to albumin, and having a small molecular size (18.6kD vs. mAbs ~150kD), which together enhance tissue penetration into the joint and could, therefore, possess an enhanced therapeutic vs. toxicity profile. The efficacy and safety of 80 mg or 40 mg every two weeks vs. placebo was assessed in 135 patients with PsA, 80% being csDMARD-inadequate responders and 13% having failed to respond to a prior TNFi. The ACR50 primary end point was met in 52% and 48% of the 80 mg and 40 mg dose groups, respectively. A greater reduction in DAS28-CRP was evident as soon as two weeks, and PASI100 clearance of skin disease in about 40% at 16 weeks in izokibep-treated patients. Adverse events were comparable among treatment groups and consistent with those observed with IL-17A inhibitors.
Izokibep is manufactured in an inexpensive E. coli system, which should allow significantly lower manufacturing cost per dose than that of a typical antibody drug. This may be its primary advantage because the efficacy and safety data do not yet suggest it may be superior to conventional monoclonal antibodies targeting IL-17A.
3. Abstract 2209
Efficacy of Secukinumab in Enthesitis-Related Arthritis and Juvenile Psoriatic Arthritis Subtypes of Juvenile Idiopathic Arthritis: Results from a Randomized, Phase 3 Study. Research by Brunner H, et al.3
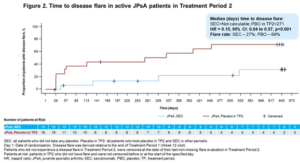
The two-year JUNIPER trial evaluated the efficacy and safety of secukinumab in patients with enthesitis-related arthritis (ERA) and juvenile PsA. The trial design was different from randomized controlled trials in adults and consisted of open-label administration of secukinumab for 12 weeks to all children and adolescents aged 2–18 with active disease defined by both three or more active joints and one or more active enthesitis sites. Those who achieved at least a JIA-ACR30 response were then randomized into the double-blinded, withdrawal period to continue secukinumab or placebo every four weeks until a disease flare or up to week 100.
For juvenile PsA patients, the flare rate was 69% in placebo patients and 27% in secukinumab-treated patients. For ERA patients, the flare rate was 45% in placebo patients and 27% in secukinumab treated patients. Improvements in JIA-ACR responses and resolution of enthesitis and dactylitis were observed in juvenile PsA patients with secukinumab treatment at week 12 and at the end of the randomized withdrawal. By the end of the follow-up, 47% of patients on secukinumab with juvenile PsA had attained inactive disease compared with 19% on placebo. Treatment was well tolerated with no new safety signals.
The impact of secukinumab vs. placebo was more impressive for juvenile PsA than for ERA patients, especially for the more stringent JIA-ACR responses (90, 100), for resolution of enthesitis and dactylitis, and for attainment of minimal disease. However, this reflects the higher rate of flare in patients with juvenile PsA vs. ERA patients randomized to placebo.
4. Abstract 1600
The Impact of Second-Line Therapeutic on Disease Control After Discontinuation of First-Line TNF Inhibitor in Patients with PsA: Analysis from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry. Research by Ogdie A, et al.4
For a patient with PsA who is failing to respond to a first-line TNFi therapy, what should be the next treatment step: cycle to an alternate TNFi, or switch to a therapy with a different mechanism of action? This important clinical question was addressed in the real-world CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry, which included patients who initiated a second-line advanced therapy (baseline) after discontinuation of first-line TNFi between May 2013 and January 2022.
Patients were divided into two cohorts: 1) 205 who initiated a second-line TNFi (i.e., cyclers), and 2) 189 who initiated a second-line non-TNFi biologic (i.e., switchers). Included patients had to have had a six-month visit to assess clinical outcomes after starting second-line treatment. Switchers had a 70% greater likelihood of achieving minimal disease activity, a nearly four times higher likelihood of achieving a Spondyloarthritis Research Consortium of Canada Enthesitis index score ≤1, a two times greater likelihood of achieving HAQ-DI ≤0.5, and a 30% greater likelihood of achieving a pain score ≤15. These differences between the two groups were all the more notable because switchers had more severe disease at baseline.
These data suggest that switching to a non-TNFi may be a superior treatment strategy than cycling to another TNFi. The reported outcomes data did not distinguish between primary and secondary failures of first-line TNFi. The patient sample was also too small to determine if there were differences among several non-TNFi therapies that are now available for PsA. Nevertheless, such pragmatic studies can provide very useful information to the clinician, especially because little is known regarding predictors of treatment response to different agents in PsA.
5. Abstract 1601
Female Psoriatic Arthritis Patients Show Differences in Treatment Response to IL12/23 Inhibition in Combination with or Without MTX Compared to Male—Results from a Multicenter Investigator-initiated Randomized Placebo-Controlled Clinical Trial. Research by Koehm M, et al.5
Subgroup analysis from different clinical trials and real-world data suggest there are differences in baseline characteristics, clinical phenotypes and treatment responses in female patients compared with males. This has been particularly well reported for responses to TNFi therapies in non-radiographic axial spondyloarthritis, in which women demonstrate a much-diminished response compared to men. This is less well studied in PsA. Gender differences on baseline characteristics and treatment responses were assessed in this investigator-initiated, randomized, placebo-controlled trial (IIT) in active PsA. Ustekinumab in combination with methotrexate, either newly initiated or ongoing, was compared with ustekinumab treatment alone.
Of the 166 patients included in this subgroup analysis of this IIT, 41.6% were women. Skin involvement (BSA, PASI) was higher in the groups of ustekinumab plus methotrexate treatment, especially in the female group, with a BSA of 9.6% and PASI of 5.3. Enthesitis was also more frequent in the female groups, whereas dactylitis was more prominent in the male groups. Other disease activity features were comparable between men and women. In comparison to the male cohort, females showed a decreased response in the resolution of pain, enthesitis, dactylitis and ACR response rates. Different from men, women do not appear to benefit from a methotrexate plus ustekinumab combinational treatment for the improvement of enthesitis and dactylitis.
A picture of gender-based differences in treatment responses in several inflammatory joint disorders is emerging that indicates women experience less benefit for pain—especially for clinically assessed enthesitis—with treatment than men. Could this reflect gender-based differences in pain hypersensitivity and/or modulation of pain perception by such factors as depression? Further studies using imaging-based assessments of joint and entheseal inflammation may provide some clarity.
6. Abstract 0292
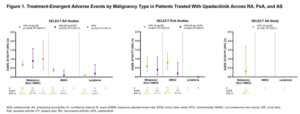
Malignancy in the Upadacitinib Clinical Trial Programs for Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis. Research by Rubbert-Roth A, et al.6
Malignancies have been associated with chronic inflammatory joint diseases, especially if poorly controlled, and with the use of immunosuppressive agents. Recent data from the Oral Surveillance study have raised concerns that the increased risk of malignancy observed with the Janus kinase (JAK) inhibitor, tofacitinib, when compared with adalimumab or etanercept, may be a class effect. The SELECT clinical trial program for upadacitinib includes active comparators in rheumatoid arthritis (RA) (i.e., methotrexate, adalimumab) and PsA (i.e., adalimumab), thereby allowing comparisons between therapeutics and across diseases.
In this report, safety data were analyzed for six RA, two PsA, and one AS trial up to June 30, 2021. Treatment-emergent adverse events (TEAEs) were reported as exposure-adjusted event rates (EAERs; events/100 patient years [E/100 PY]). Age-gender adjusted standard incidence ratios (SIR) were calculated for RA, due to a sufficient number of reports, using the SEER database for the general population.
The safety data analysis was based on 4,298 patients (RA, n=3209; PsA, n=907; AS, n=182) and 11,271.5 PY of exposure. In RA, event rates of malignancy excluding nonmelanoma skin cancer (NMSC) were similar between 15 mg of upadacitinib, adalimumab and methotrexate; rates of NMSC were higher with 15 mg of upadacitinib compared with adalimumab and methotrexate. The most common types of malignancy excluding NMSC were breast cancer and lung cancer.
In PsA, rates of NMSC and malignancy excluding NMSC were similar between 15 mg of upadacitinib and adalimumab. In AS, a single event of malignancy excluding NMSC was reported with 15 mg of upadacitinib affecting the tongue in a long-term smoker. Being older and having higher BMI were associated with increased risk in patients with PsA.
Being older, male and in the U.S., compared with other regions, were associated with an increased risk of malignancy excluding NMSC in patients with RA, while being older and having higher BMI were associated with increased risk in patients with PsA.
These data are reassuring for clinicians, but PsA and AS patient numbers are small and duration of follow-up is only two to three years, compared with the 4.4 years in the Oral Surveillance study.
7. Abstract 0510
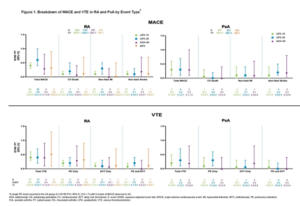
MACE and VTE Across Upadacitinib Clinical Trial Programs in Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis. Research by Charles-Schoeman C, et al.7
Major adverse cardiovascular events (MACE) and venous thromboembolic events (VTE) have been associated with chronic inflammatory joint diseases, especially if poorly controlled, and recent data from the Oral Surveillance study have raised concerns that the increased risk of MACE and VTA observed with the Janus kinase inhibitor, tofacitinib, when compared with adalimumab or etanercept, may be a class effect.
The SELECT clinical trial program for upadacitinib includes active comparators in rheumatoid arthritis (RA) (i.e., methotrexate, adalimumab) and PsA (adalimumab) thereby allowing comparisons of these events between therapeutics and across diseases.
In this report, safety data were analyzed for six RA, two PsA and one AS trial up to June 30, 2021. TEAEs of MACE (e.g., cardiovascular death, non-fatal myocardial infarction and non-fatal stroke) and VTE (e.g., pulmonary embolism and deep vein thrombosis) were adjudicated by a blinded, independent cardiovascular adjudication committee. Time to event was analyzed using the Kaplan-Meier method, and EAERs were evaluated over six-month increments.
Across trials, 4,298 patients received one or more dose(s) of 15 mg of upadacitinib and 2,125 received 30 mg of upadacitinib, 1,008 patients received 40 mg of adalimumab, and 314 patients received methotrexate. At baseline, 40–50% of patients had two or more cardiovascular risk factors, and the proportion of patients 65 years or olde ranged from 6–23%.
Of the 41 MACE reported in the 15 mg upadacitinib group across RA and PsA, only two RA patients did not have one or more cardiovascular risk factors at baseline. There were two fatal VTEs across trials, both occurring in the RA 15 mg upadacitinib group. Overlapping confidence intervals were observed across UPA doses and comparators for rates of MACE, as well as for rates of VTE in both RA and PsA. In univariable analyses, factors potentially associated with MACE or VTE occurrence in patients with RA receiving 15 mg of upadacitinib included age 65 years or older; baseline hypertension; diabetes mellitus; smoking; history of cardiovascular event or VTE; as well as use of aspirin, statins or anti-thrombotics. In PsA, aspirin use was associated with increased risk of MACE.
As for the assessment of malignancy, the data for MACE and VTE for patients receiving 15 mg of upadacitinib daily in the SELECT trial program do not raise any red flags, although patient numbers are small in the PsA and AS programs and duration of follow-up is relatively short.
8. Abstract 1235
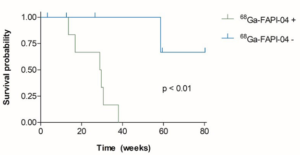
Fibroblast Activation in Psoriasis Patients Assessed by 68Ga-FAPI-04 PET-CT Is Associated with Progression to Psoriatic Arthritis. Research by Fagni F, et al.8
Subclinical inflammatory lesions may occur in patients with cutaneous psoriasis and have been shown to predict development of PsA. However, accurate prediction of such progression remains challenging, and there is no serological biomarker that has this predictive capacity. There is enhanced activation of synovial and entheseal fibroblasts in PsA and so it seemed reasonable to hypothesize that demonstration of increased fibroblastic activity in joints and/or entheses using a tracer visible on PET-CT imaging could help identify patients with psoriasis at risk of progression to PsA. 68Ga-labeled selective inhibitor of fibroblast activation protein-α (68Ga-FAPI-04) is a radiotracer targeting FAP that permits in vivo and non-invasive visualization of fibroblast activity on PET-CT.
Ten patients with psoriasis but without clinical evidence of synovitis, enthesitis, dactylitis or axial inflammation had 68Ga-FAPI-04 PET-CT and were followed up. Seven patients showed 68Ga-FAPI-04 uptake at synovio-entheseal sites (i.e., finger joints, shoulders, knees, tarsus, lumbar facet joints and interspinous ligaments, greater trochanter, ischial tuberosity) and six progressed to PsA after median follow-up of 207 days. All three patients with negative imaging developed no signs of PsA after a median follow-up of 301 days.
This technique looks promising for the investigation of arthralgias in patients with psoriasis, but it requires further assessment in larger prospective cohorts to determine how accurately it predicts development of PsA as opposed to other sources of joint pain, such as osteoarthritis. Its performance should also be compared with imaging modalities, such as ultrasound and MRI, which are more readily available.
Kaplan–Meier plots of PsA-free survival according to the presence or absence of any synovio-entheseal 68Ga-FAPI-04 uptake (P<0.01).
9. Abstract 1602
Fibroblast Activation Protein (FAPP PET-CT for Depicting Inflammatory Joint Damage in Patients with Psoriatic Arthritis. Research by Ramming A, et al.9
Predictors of joint damage in PsA are currently lacking, but it is known that substantial mesenchymal tissue activation occurs in patients with PsA, which may lead to structural damage following activation of fibroblasts and secretion of metalloproteinases. A new development reported at this year’s meeting is the use of tracers that bind fibroblast activation protein (FAP) and allow recognition of activated mesenchymal cells by positron-emission tomography (PET).
The uptake of a 68Ga-labeled FAP-inhibitor tracer was analyzed in both peripheral and axial joints of patients with PsA and healthy controls. Hand and spine MRI scans were performed in parallel to assess inflammation and structural lesions. Follow-up assessment was conducted in a subset of patients following treatment with TNF or IL-17A inhibitors. The tracer accumulated at sites of synovitis and enthesitis in patients with PsA, but not healthy controls, and the degree of accumulation correlated with the degree of pain and disease activity. The degree of tracer uptake at baseline predicted the development of joint damage on hand MRI.
Treatment with cytokine inhibitors reduced FAP expression, which was associated with the prevention of joint damage on MRI, while persistence of expression predicted progression of joint damage. Synovial tissue biopsies from FAP+ lesions revealed FAP+ fibroblasts, which reverted from an aggressive damage-inducing phenotype to an inert form in treated patients.
This imaging biomarker should be further validated as a prognostic indicator of joint damage. There is a major unmet need for more responsive end points reflecting structural damage in PsA that could be used in clinical trials of disease-modifying therapies.
10. Abstract 2256
Increased Brain Functional Connectivity to the Insula Is Associated with Nociplastic Pain in Psoriatic Arthritis. Research by Sunzini F, et al.
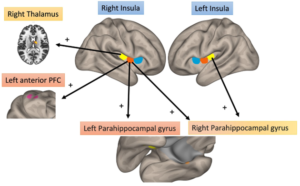
Pain hypersensitivity is an established feature of PsA, as exemplified by the frequent co-existence of fibromyalgia. Such pain is now termed as being nociplastic and abnormalities indicative of increased connectivity have been reported on functional MRI in the insular cortices (IC), the key sensory integrative hubs that capture and compute distinct dimensions of pain perception. In this report, nociplastic pain was assessed in PsA patients using the ACR 2011 Fibromyalgia Survey criteria followed by a six-minute functional MRI scan. Functional MRI connectivity was assessed in the anterior, middle (mid) and posterior (post) IC and associations with the degree of nociplastic pain assessed by regression adjusted for age and gender.
Of 46 patients with PsA, 41% fulfilled criteria for fibromyalgia. Higher fibromyalgia scores displayed increased connectivity between: 1) right, mid-IC to left, anterior prefrontal cortex, 2) right, post-IC to right thalamus, and 3) left, post-IC to right parahippocampal gyrus.
This report presents the first neurobiological evidence of nociplastic pain in PsA and is characterized by increased functional brain connectivity between the mid-posterior IC and brain areas involved in sensory (thalamus), learning/affective (parahippocampal gyrus) and cognitive (prefrontal cortex) pain modulation.
Now that we can better document this phenomenon in patients with PsA, the question emerges, “How can rheumatologists intervene to help alleviate this type of pain?”
 Walter P. Maksymowych, FRCP(C), is chief medical officer of CARE Arthritis Ltd., Alberta, Canada. He is also a professor and medical scientist in the Department of Medicine, Division of Rheumatology, University of Alberta. He is the 2012 recipient of the Distinguished Investigator Award from the Canadian Rheumatology Association.
Walter P. Maksymowych, FRCP(C), is chief medical officer of CARE Arthritis Ltd., Alberta, Canada. He is also a professor and medical scientist in the Department of Medicine, Division of Rheumatology, University of Alberta. He is the 2012 recipient of the Distinguished Investigator Award from the Canadian Rheumatology Association.
Disclosures
Dr. Maksymowych has acted as a paid consultant and participated in advisory boards for AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Galapagos, Janssen, Novartis, Pfizer and UCB; received research and/or educational grants from AbbVie, Novartis, Pfizer and UCB; and received speaker fees from AbbVie, Janssen, Novartis, Pfizer and UCB.
References
- Merola J, Landewé R, McInnes I, et al. Bimekizumab treatment in patients with active psoriatic arthritis and inadequate response to tumor necrosis factor inhibitors: 16-week efficacy and safety from a phase 3, randomized, double-blind, placebo-controlled study [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- Behrens F, Taylor P, Mease P, et al. Achievement of different treatment targets with izokibep demonstrates efficacy benefits in patients with active psoriatic arthritis: Results from a 16-week randomized, placebo-controlled phase 2 clinical trial [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- Brunner H, Chertok E, Dehoorne J, et al. Efficacy of secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis subtypes of juvenile idiopathic arthritis: Results from a randomized, phase 3 study [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- Ogdie A, McLean R, Blachley T, et al. The impact of second-line therapeutic on disease control after discontinuation of first line TNF inhibitor in patients with PsA: Analysis from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- Koehm M, Foldenauer A, Rossmanith T, et al. Female psoriatic arthritis patients show differences in treatment response to IL12/23 inhibition in combination with or without MTX compared to male—Results from a multicenter investigator-initiated randomized placebo-controlled clinical trial [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- Rubbert-Roth A, Kakehasi A, Takeuchi T, et al. Malignancy in the upadacitinib clinical trial programs for rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- Charles-Schoeman C, Choy E, et al. MACE and VTE across upadacitinib clinical trial programs in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- Fagni F, Simon D, Kleyer A, et al. Fibroblast activation in psoriasis patients assessed by 68Ga-FAPI-04 PET-CT is associated with progression to psoriatic arthritis [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- Rauber S, Mohammadian H, Schmidkonz C, et al. Fibroblast activation protein (FAP) PET-CT for depicting inflammatory joint damage in patients with psoriatic arthritis [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- Sunzini F, Stefanov K, Al-Wasity S, et al. Increased brain functional connectivity to the insula is associated with nociplastic pain in psoriatic arthritis [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).