Drug class: CLTA4-Ig
Drug: abatacept (Orencia)
Adverse reactions: The most common adverse reactions (≥10%) are headache, upper respiratory tract infection, nasopharyngitis and nausea.
Drug class: IL-12/23 inhibitor
Drug: ustekinumab (Stelara)
Adverse reactions: The most common adverse reactions (≥3%) are arthralgias and nausea.
Drug class: JAK inhibitor
Drug: tofacitinib (Xeljanz), upadacitinib (Rinvoq)
Boxed Warning: Serious Infections, Mortality, Malignancy and Thrombosis
- There is an increased risk of serious infections leading to hospitalization or death, including TB and bacterial, invasive fungal, viral, and other opportunistic infections.
- If a serious infection develops, discontinue treatment until the infection is controlled.
- Before starting treatment, perform a test for latent TB; if positive, start treatment for TB prior to starting the drug. Monitor all patients for development of active TB during treatment, even if the initial latent TB test was negative.
- Thrombosis, including pulmonary embolism, deep venous thrombosis and arterial thrombosis have occurred in patients treated with JAK inhibitors.
- Malignancies have been observed in patients treated with JAK inhibitors.
Adverse reactions: The most common adverse reactions (≥2%) are upper respiratory tract infection, nasopharyngitis, diarrhea and headache.
Drug class: IL-17 receptor antagonist
Drug: brodalumab (Siliq)—off-label, ixekizumab (Taltz), secukinumab (Cosentyx)
Boxed Warning for Brodalumab (Siliq): Suicidal Ideation and Behavior
- Suicidal ideation and behavior, including completed suicides, have occurred in patients treated with brodalumab.
- Prior to prescribing, weigh potential risks and benefits in patients with a history of depression and/or suicidal ideation or behavior.
- Patients with new or worsening suicidal thoughts and behavior should be referred to a mental health professional.
- Advise patients and caregivers to seek medical attention for manifestations of suicidal ideation or behavior, new onset or worsening depression, anxiety, or other mood changes.
- Brodalumab is available only through a restricted program called SILIQ REMS.
Adverse reactions: The most common adverse reactions (≥1%) are infections, nasopharyngitis and headaches.
Drug class: IL-23 inhibitor
Drug: guselkumab (Tremfya), risankizumab-rzaa (Skyrizi), tildrakizumab-asmn (Ilumya)—off label
Adverse reactions: The most common adverse reactions (≥1%) are upper respiratory infections, headache, fatigue, injection-site reactions and diarrhea.
IN SUM
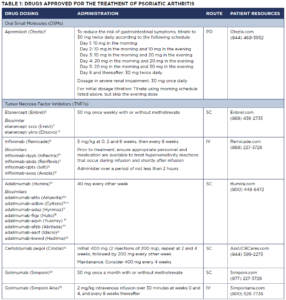
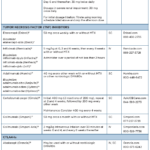
See Table 1 for administration and other information for all of these drugs. Important note: All of these treatments carry the risk of severe side effects. Please refer to the full prescribing information (as indicated in the References section) for each drug.
Mary Choy, PharmD, BCGP, FASHP, is a medical writer and editor living in New York City. Dr. Choy is the co-author of Healthcare Heroes: The Medical Careers Guide.