Leeds has continued its reputation as a leading center for research in PsA, and the infirmary has expanded on Dr. Wright’s legacy. In the 1990s, a young Irish physician journeyed to Leeds, and some years later his Dublin mentor commented, “Always had a big brain, that boy.” Dennis McGonagle, PhD, perceptively and convincingly revitalized the fundamental paradigms in these disorders. He partnered with Mike Benjamin, PhD, MD, an anatomist from Cardiff, and they have performed seminal studies on the enthesis and its central role in the pathogenesis of spondylitis.9
Shortly afterwards, Will Taylor, PhD, a visiting fellow from New Zealand, was instrumental in assembling the first international collaboration to develop new classification criteria for PsA.10 This latter initiative led to the formation of a more permanent group of investigators with strong interests in the science and clinical aspects of PsA. The impetus for this effort was provided by Philip Mease, MD, of Seattle Rheumatology Associates, and Dafna Gladman, MD, in Toronto Western Hospital in Ontario, Canada. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) currently has more than 300 members worldwide, and this organization has pioneered a number of important initiatives related to outcome assessment and treatment recommendations, and has improved collaboration between rheumatologists and dermatologists in the clinical and research domains.11,12
Despite Dr. Wright’s robust contributions to the literature in his studies on PsA, the publication record on this subject was relatively scant. A review of Medline publications between 1970 and 1990 reveals that 128 manuscripts were published on PsA compared with 23,926 on RA during the same time span. Scientific studies during this era focused on genetic associations with class I major histocompatibility complex (MHC) alleles and clinical trials with agents such as 1.25-dihydroxyvitamin D, gold compounds, interferon-γ, and methotrexate. For the most part, these trials were underpowered or open label, and a small number of subjects were enrolled. To complicate matters, outcome assessments were not standardized or validated. PsA had definitely not hit the mainstream in the rheumatology community. However, in the late 1990s, the field of research changed dramatically with the arrival of new laboratory techniques and a novel class of therapeutic agents.
TABLE 2: Historical and Clinical Landmarks in Psoriatic Arthritis
- Genetics
- Association with MHC class I alleles, IL-1 cluster, TNF, IL-23R, AND IL-12
- IL-23R and CW-6 have stronger links to PsA and MICA stronger association with PsA
- Histopathologic features of psoriatic synovium
- Increased vascularity
- Histologic features more similar to other PsA than RA
- Absence of antibodies to shared epitope
- Elevated levels of TNF in the synovial tissue and fluid
- TH1 and possibly TH17 immune response
- Enthesitis
- A site of inflammation or origin of disease?
- Synovial–enthesial complex comprised of multiple structures subject to biomechanical stress and inflammation
- Bone marrow edema
- Aberrant bone remodeling
- Overexpression of RANKL and increased levels of circulating OCP
- Bone resorption fueled by TNF
- Increased pathologic bone formation linked to BMP molecules and possibly the Wnt signaling pathway
- Psoriatic disease
- Clustering of disorders including psoriasis, arthritis, uveitis, inflammatory bowel disease, obesity, metabolic disorders, and cardiovascular disease
- Prominent contribution from monocyte effector cells
Nurture Versus Nature
Evidence supporting a link with MHC class 1 alleles in PsA was published soon after the association of HLA-B27 with ankylosing spondylitis was established. A major impediment to the study of genetics in PsA is the need to separate patients with joint and skin manifestations. Recent data using genome-wide association scans have identified a number of genes that segregate with PsA, although it appears that Cw6 and IL-23 have stronger links with the skin disease, while major histocompatibility complex class I chain-related gene A (MICA) alleles are associated more closely with the arthritis.13 Much interest has focused on the contribution of infections and trauma, which may favor the development of arthritis, although the importance of these environmental factors is not well established.14
Synovial Studies
The study of psoriatic synovium was ushered in by the findings of Espinoza et al. that revealed unique histopathologic and ultrastructural findings in the blood vessels traversing the synovium.15 Subsequent research by Reese et al. in Leeds followed by studies from De Rycke et al in Belgium showed that PsA synovium had histopathologic features that were more akin to other forms of PsA than RA, particularly in regards to the presence of neutrophils, prominent vascularity, and lining layer hyperplasia.16,17 Additional studies have identified a plethora of cytokines; the most prominent are tumor necrosis factor (TNF), vascular endothelial growth factor, and interleukin (IL)-1. Although analysis of psoriatic skin has uncovered a central role for IL-17 and IL-22 in cutaneous inflammation, the importance of Th17 cells in PsA is a matter of debate.18 Synovial studies focused on IL-17 have not been published, and a recent clinical trial with ustekinumab, a molecule that blocks both IL-12 and IL-23, was rather unimpressive compared with the clinical trial data with anti-TNF agents.19
Enthesis: Interesting Linchpin or Focus Inflammation?
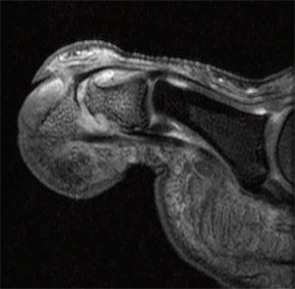
Imaging studies performed by Dr. McGonagle and others uncovered widespread signals in the bone marrow in PsA subjects. They noted focal bone marrow edema (BME) adjacent to entheseal insertions and more generalized BME at distant sites in some patients. They also noted the close proximity of entheseal attachments to other important structures such as synovium, bursa, and fibrocartilage. Based on these observations, they proposed the concept of the synovio-entheseal (SEC) complex to explain the origins of psoriatic joint and tissue inflammation.20 In this model, the interaction between local mechanical forces and structures in the SEC trigger cytokine release via an innate immune response, and these cytokines promote tissue inflammation. This model combines dysfunction at the local level with an aberrant inflammatory response. Other investigators acknowledge an important contribution of the entheses, but they challenge the concept that this structure is the initial focus of inflammation. The presence of osteitis is often striking on MRI in PsA patients and studies, but the pathologic correlate of this radiographic term has been controversial. MRI imaging and histopathologic studies of arthritic TNF transgenic mice revealed that BME represents expansion of monocytes in the bone marrow (see Figures 1A, p. 19, and 1B, p. 20) that corresponds with BME signals on MRI.21
Aberrant Bone Marrow Remodeling
One of the most intriguing features of PsA is the tendency for marked bone resorption and pathologic new bone formation.22 Moreover, this aberrant remodeling can take place in the peripheral joints and axial skeleton. Histopathologic studies revealed high receptive activator of nuclear factor kappa–B ligand (RANKL) expression in PsA synovium and elevated levels of osteoclast precursors (OCP) in the circulation of PsA patients. The OCP are exquisitely sensitive to TNF blockade, and levels of these cells are increased in some psoriasis patients without musculoskeletal symptoms, suggesting that these cells may be susceptibility markers for arthritis, although additional confirmation is required. Recent studies have uncovered a number of possible pathways that lead to pathologic bone formation, but emphasis is now centered on bone morphogenetic proteins (BMP) and other molecules that modulate the Wnt signaling pathway, such as Dickkopf (DKK-1) and sclerostin.23
Psoriatic Disease
In the majority of patients, psoriasis precedes arthritis by about 10 years. Epidemiologic studies have shown a high prevalence of obesity that is an incident risk factor for psoriasis.24 It has also become apparent that patients with psoriasis often manifest associated disorders at a higher prevalence than controls. Recently described mechanisms that link obesity and inflammation raise the possibility that obesity may contribute to psoriasis onset in patients with certain genetic backgrounds. Other disorders, besides arthritis, that aggregate with psoriasis are diabetes, metabolic syndrome, inflammatory bowel disease, and premature atherosclerosis.25 A contributing role for monocyte effector cells in fostering disease at these disparate sites has been proposed.
![Figure 1B: Histologic (hematoxylin and eosin stain [H&E]; left panels) and MRI features (right panels) in TNF transgenic (Tg) mice and wildtype (WT) littermates without the transgene. Note the striking bone marrow edema in the Tg mouse. This finding correlates with an expansion of red marrow as shown. Staining of the cells revealed an expansion of CD11b+ monocytes but not lymphocytes.](https://www.the-rheumatologist.org/wp-content/uploads/springboard/image/THR_2009_08_pp20_01.jpg)
Lessons from Clinical Trials
As outlined in this article, great advances have occurred in our knowledge of some of the mechanisms that are responsible for PsA. Advances at the bench, however, have been accompanied by remarkable improvements in therapy for PsA and psoriasis. The TNF inhibitors have proven to be extremely effective for both the skin and joint disease in many patients.26 Surprisingly, anti–T cell agents such as efalizumab and alefacept have proven modestly effective for psoriasis but not for PsA, and efalizumab was recently recalled due to concerns over progressive multifocal leukoencephalopathy. Other agents that have potential for the treatment of PsA aside from ustekinumab are abatacept, rituximab, anti-RANKL antibody, and antibodies to IL-6R and IL-17. In many cases, financial support from pharmaceutical companies fueled the advance of clinical trial design and outcome measures and in some cases supported studies focused on disease mechanisms.



