 Corticosteroids still represent the mainstay of treatment of patients with active disease. They have been used for more than 60 years, and although prolonged use is associated with organ damage, they have been shown to be lifesaving in various phases of the history of the disease.
Corticosteroids still represent the mainstay of treatment of patients with active disease. They have been used for more than 60 years, and although prolonged use is associated with organ damage, they have been shown to be lifesaving in various phases of the history of the disease.
History of Use
First introduced in the late 1960s in renal transplantation, high-dose intravenous therapy was shown to be safe and effective in increasing graft survival in patients treated with one to three grams of intravenous methylprednisolone.1,2
In 1974, I remember that Edgar Cathcart, one of my mentors, and I were talking with two nephrologists in the cafeteria of Boston University Hospital about their results with pulse therapy in allograft rejection, and the idea came up to try to abrogate acute deterioration of renal function in lupus patients with pulse therapy. The initial results in the first seven patients were published in January 1976.3
As one of the authors of the original paper, I review here the events that surrounded the use of this therapy, as published in The Lancet 40 years ago (see Figure 1, below). We found that in five of seven SLE patients with biopsy-proved diffuse proliferative glomerulonephritis, a marked improvement of renal function was found after three days of 1.0 g pulse of intravenous of methylprednisolone (see Figure 2, below).
Figure 1: The First Paper on Systemic Lupus Erythematosus Nephritis Published
Cathcart ES, Scheinberg MA, Idelson BA, Couser WG. Beneficial effects of methylprednisolone ‘pulse’ therapy in diffuse proliferative lupus nephritis. Lancet. 1976 Jan 24;307(7952):163–166.
Abstract: Seven patients with diffuse proliferative lupus nephritis were subjected to high-dose intravenous methylprednisolone (pulse) therapy. Following the pulse, five patients with rapidly deteriorating renal function improved within three days and their serum-creatinine levels returned to baseline by one month. All seven patients demonstrated reversal of severe immunological abnormalities including increased serum D.N.A. binding, decreased serum C3 levels, and reduced number of T lymphocytes in the peripheral blood. This form of therapy may make it possible to maintain patients with lupus nephritis on lower doses of steroids than is normally feasible.
Since the publication of the original paper in 1976, bolus injections of steroids have seen considerable variation in the dose, number, timing and duration of higher doses. Despite 40 years of use, the clarity of the mechanism of action is still, to some extent, unknown. It is well known that pulse therapy is cumulatively less toxic than treatment with continuous oral steroids at lower doses. However, it is also known that pulse therapy may be associated with side effects, and is contraindicated in systemic infections and uncontrolled hypertension. Its use can lead to metabolic disturbances and changes in behavior, requiring adequate monitoring during its use.
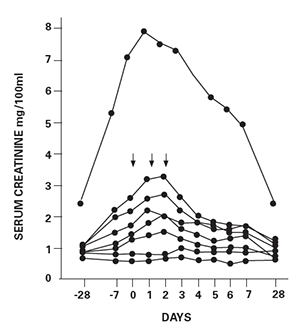
Figure 2
Ten years later, in 1986, the group at the National Institutes of Health reported the long-term results of its trial with monthly pulses of cyclophosphamide and later on, in further trials, confirmed the beneficial effects of high-dose steroids by combining pulses of cyclophosphamide with steroid pulses.
Changing Views
It seems that, in the next decade, attempts to minimize steroid use in patients with systemic lupus erythematosus (SLE) and replacing it with B cell depletion are starting to grow, but even in that scenario, in the beginning, patients will still receive a limited number of pulse steroids. In a just-released paper, 69 SLE experts indicated their preferences to treat serious lupus nephritis, and high-dose steroids are still the first line of treatment, followed by mycophenolate.
In the next decade, it appears that biologics will be included in the standard of care. Currently, the only approved biologic drug for SLE is belimumab. Steroid pulse therapy is expected to continue to be used in periods of exacerbations.
The use of steroid pulse therapy since its publication has gained increased popularity in various clinical autoimmune disease states and is still an option for therapy after 40 years (see Table 1, below).
| Table 1: Pubmed Search 1976–2015 | |
| Lupus nephritis and pulse therapy |
366 papers |
| Lupus disease and pulse therapy |
1,191 papers |
| Autoimmune disease and pulse therapy |
4,708 papers |
 Morton Scheinberg, MD, PhD, is an internist and rheumatologist at the Hospital Israelita Albert Einstein in São Paulo, Brazil, and director of clinical research at Hospital AACD, also in São Paulo. He received his PhD in immunology from Boston University, a Free Associate Professor from the Universidade São Paulo, and is an ACR Master.
Morton Scheinberg, MD, PhD, is an internist and rheumatologist at the Hospital Israelita Albert Einstein in São Paulo, Brazil, and director of clinical research at Hospital AACD, also in São Paulo. He received his PhD in immunology from Boston University, a Free Associate Professor from the Universidade São Paulo, and is an ACR Master.
References
- Kauntz SL, Cohn R. Initial treatment of renal allografts with large intrarenal doses of immunosuppressive drugs. Lancet. 1969 Feb 15;293(7590):338–340.
- Woods JE, Anderson CF, Deweerd JH, et al. High-dosage intravenously administered methylprednisolone in renal transplantation. A preliminary report. JAMA. 1973 Feb 19;223(8):896–899.
- Cathcart ES, Scheinberg MA, Idelson BA, Couser WG. Beneficial effects of methylprednisolone ‘pulse therapy in diffuse proliferative lupus nephritis. Lancet. 1976 Jan 24;307(7952):163–166.
- Austin HA 3rd, Klippel JH, Balow JE, et al. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986 Mar 6;314(10):614–619.
- Illei GG, Austin HA, Crane M, et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med. 2001 Aug 21;135(4):248–257.
- Lightstone L. Minimising steroids in lupus nephritis—will B cell depletion pave the way? Lupus. 2013 Apr;22(4):390–399.
- Condon MB, Ashby D, Pepper RJ, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. 2013 Aug;72(8):1280–1286.
- Muangchan C, van Vollenhoven RF, Bernatsky SR, et al. Treatment algorithms in systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2015 Sep;67(9):1237–1245.
- Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011 Dec;63(12):3918–3930.
- Scheinberg M, de Melo FF, Bueno AN, et al. Belimumab for the treatment of corticosteroid-dependent systemic lupus erythematosus: From clinical trials to real-life experience after 1 year of use in 48 Brazilian patients. Clin Rheumatol. 2016 Jul;35(7):1719–1723.
- Scheinberg M. The history of pulse therapy in lupus nephritis (1976–2016). Lupus Sci Med. 2016 Apr 8;3(1):e000149.
