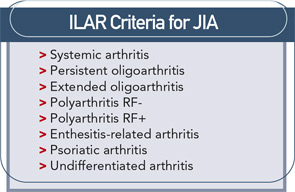
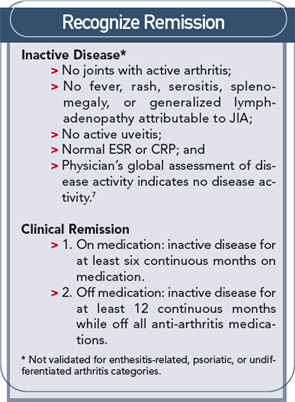
Treatment of juvenile idiopathic arthritis (JIA) has a straightforward goal: to achieve disease remission and allow children to grow and develop normally. Several challenges stand in the way. There is a lack of decisive data on the medication (or combination of medications) that can achieve this xaa reliably and the etiology of disease is unknown. The most important targets for therapy among the myriad of documented immunologic abnormalities are not certain.
It is clear that we need more effective treatment because many studies demonstrate that most children with JIA have persistent disease. According to studies of children with JIA with four to 20 years of follow-up, the majority of children with extended oligoarthritis, polyarthritis, and systemic onset arthritis will spend two-thirds of their disease course with active disease and ongoing synovitis and enter adulthood with ongoing disease.1
Slim Data on JIA
Published clinical trials of new biologic therapies and methotrexate have focused on improvement of JIA, not disease remission. However, retrospective reports have shown that methotrexate alone or in combination with prednisone, intra-articular steroids, hydroxychloroquine, or sulfasalazine can result in remission in 20% to 45% of patients at an average of 13 months after treatment initiation.2 Data from more recent clinical trials for biologic therapies in JIA suggest that 19% to 38% of patients can achieve a state of no active joints and/or a physician global evaluation of zero.3 Unfortunately, the remission achieved is often short lived, with the majority of children flaring after three years off of medications and only 6% with disease-free, medication-free survival past five years.1
Many investigations have demonstrated superior outcomes for adult patients with RA who receive early treatment, prompting speculation on a window of opportunity during which aggressive therapy can have a profound long-term effect. It is reasonable to presume that this would also be true for JIA.
Because no one drug or combination of drugs have been identified as the best treatment for JIA, the most important aspect of treating JIA is the conceptual framework of rapidly achieving inactive disease and, hopefully, disease remission.
It is crucial to achieve inactive or remission disease states because ongoing synovitis can allow damage to cartilage and bone and perpetuate and expand the inflammatory process. MRI scans have shown the presence of erosive disease within just a few months of disease onset for some children. Routine X-rays also demonstrate that the greatest amount of joint destruction occurs within the first few years of disease.4 There is also the psycho-social impact of active disease on a child and the family to consider. While altering activities for weeks and months may not be significant for adults, missing a season of orchestra, dance, soccer, basketball, or cheerleading can have a large impact on a child.
The current strategy for JIA treatment should be early, rapid, and persistent treatment of initial disease and all disease flares to achieve inactive disease in all patients.
Ideally, a clinician could use laboratory or other biological data to determine which patients would respond to which medications or combinations of medications. Unfortunately, except for rheumatoid factor (RF) positivity (and probably anticyclic citrullinated peptide antibody positivity), there are no markers of JIA that identify those children who will have persistent or difficult-to-treat disease. No investigations have found biologic differences at the onset of disease between those children who appear to have mild disease and those with more painful symptoms, or between those children who will develop joint damage and those who do not. Thus, the current strategy for JIA treatment should be early, rapid, and persistent treatment of initial disease and all disease flares to achieve inactive disease in all patients. Potential side effects must be carefully monitored with appropriate laboratory tests. Below is an overview of treatments to be used (most often in combination) to ameliorate disease.
NSAIDs
These drugs can decrease morning stiffness and joint pain and increase mobility in some patients. By the time they first see a rheumatologist, most children will have been treated with one or more nonsteroidal anti-inflammatory drugs (NSAIDs). This approach can be continued if it has caused significant improvement. Because of their high potential for side effects, these drugs are usually the first to be tapered and discontinued when a patient achieves inactive disease.
Corticosteroids
These are very helpful for many patients.
Intra-articular steroids are frequently used for children with only a few joints involved. In the case of multiple joint involvement, multiple joints may be injected when they are particularly problematic or interfering with function. Injections can be time consuming by interrupting a busy clinic for procedures, or having to be performed under anesthesia or performed by interventional radiology. However, for the majority of patients, steroid injections are very effective and can be repeated multiple times per year in a specific joint.
Oral prednisone/prednisolone can be particularly effective in gaining control of polyarthritis while other disease-modifying antirheumatic drugs are becoming effective. Start with a 0.5–1 mg/kg/day dose for several weeks, with gradual taper to 0.15 mg/day for several months; this does not appear to result in long-term toxicity. Patients and their families must be counseled about potential weight gain or behavioral abnormalities and the need for extra calcium and vitamin D. Recent investigations point to active disease – rather than low-dose steroid use – as the strongest predictor of osteoporosis.
If a patient is hospitalized, IV pulse methylprednisolone (30 mg/kg/day for three days with 1-gram maximum) can quickly decrease disease activity. Many patients will benefit from weekly outpatient infusions while other medications begin to be effective.
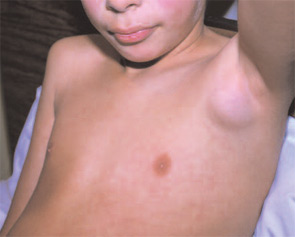
Methotrexate
This is still the mainstay of therapy for the majority of children with JIA. After administration, methotrexate (MTX) has a relatively short half-life in plasma; 80% to 90% of methotrexate is cleared by the kidneys in less than 24 hours. Because children with JIA usually have excellent glomerular filtration, MTX is cleared rapidly.
The single weekly doses of MTX found to be effective in children, 0.3–1.0 mg/kg (10–30 mg/m2), are higher than weekly doses usually reported for adult patients. The route of administration is an important factor for children, because these doses are in the range in which oral MTX becomes increasingly less well absorbed: 0.3 mg/kg (≥10 mg/m2). For children, absorption is best when MTX is given without food. The amount of MTX absorbed by children has wide interpatient variability. For these reasons, subcutaneous administration may be more effective than oral MTX. Experience leads many clinicians to change to subcutaneous MTX at dosages of 0.5 mg/kg or higher, for better absorption and fewer gastrointestinal (GI) side effects. Parents and teenagers can easily be taught to administer subcutaneous MTX at home. A parenteral solution of MTX (25 mg/ml) taken orally is commonly used for pediatric patients when liquid oral dosing is desired.
MTX rapidly enters cells, where it is polyglutamated by hepatocytes, red cells, fibroblasts, bone-marrow myeloid precursors, and possibly other cells. Polyglutamated methotrexate accumulates intracellularly and may explain the emergence of nausea and vomiting in patients after several years of treatment. The hope was that levels of intracellular polyglutamated MTX will correlate with response to MTX, but that has not been the case to date.
MTX is well tolerated by children and does not appear to be more toxic than NSAIDs, if monitored properly. Common side effects in children include elevation of serum aminotransferase levels and nausea. Transient mucositis can occur, while hematologic abnormalities are uncommon, and there are reports of headache, alopecia, gastric ulcer, and mood changes. There are multiple strategies for decreasing side effects, the most important being recognition of the potential role of NSAIDs. Many side effects from NSAIDs are similar to those of MTX. Withholding a dose of NSAID before and/or after MTX administration, changing the NSAID, or discontinuing it may improve MTX tolerance. When faced with GI symptoms in an individual, try giving MTX at bedtime, changing MTX to subcutaneous, or administering an antiemetic before MTX administration. If these strategies fail, the dose of MTX may need to be lowered.
Because folic acid depletion is thought to contribute to MTX side effects, most clinicians prescribe a daily vitamin with folate or folate 1 mg/day.
Other side effects to consider are:
- Pneumonitis is rarely reported in children treated with MTX for rheumatic diseases;
- Cirrhosis has not been reported in children using MTX for rheumatic diseases;
- Oncogenic potential is low. There are only rare reports of lymphoma in patients with JIA. Reports in adults with RA may be Epstein-Barr virus–related or related to the background risk from persistently active disease;
- Gonadal function and reproduction are not altered by MTX. However, both males and females should wait for three months after discontinuing MTX before trying to conceive because MTX is a powerful teratogen. Young women receiving MTX should repeatedly be reminded to use effective birth control.
Leflunomide
After absorption, leflunomide is metabolized into an active metabolite which reaches peak levels between six and 12 hours. The most common adverse reactions are gastrointestinal symptoms: diarrhea, anorexia, abdominal pain, dyspepsia, gastritis, and elevation of transaminases. Other problems occurring in approximately 5% to 10% of patients include rash/allergic reactions, headache, and reversible alopecia. Less common are weight loss and hypophosphatemia. Neither the prodrug nor the active metabolite are dialyzable. Elimination can be hastened by the use of cholestyramine. Leflunomide is teratogenic. There are no long-term studies to assess its carcinogenicity or effect on fertility.
Cyclosporine
This is uniquely effective for treating macrophage activation syndrome (MAS) (see p.27 for more on MAS). Use 3 to 5 mg/kg intravenously, and change to oral dosing when disease is controlled.
Biologic Therapies
Etanercept, infliximab, adalimumab, anakinra, abatacept, and rituximab have all been demonstrated or reported to be effective in treating JIA. Their use in children poses special problems, including the increased risk of infections (especially varicella); uncertainty about the timing and performance of the usual immunizations; concerns about the potential long-term effects of biologic medications on younger children with developing immune systems; and the possibilities of later malignancies or possible development of central nervous system demyelinating disease.
All children should have documented negative tuberculosis (TB) skin tests before starting any biologic therapy, and live vaccines are contraindicated while patients are receiving biologic therapy.
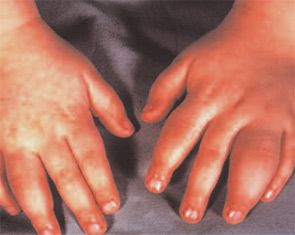
Anti-TNF Agents
TNF blockers have been documented to be very effective treatments for JIA. Prospective investigations have been done on patients with polyarticular course of disease with oligo, poly, or systemic onset.
By three months of treatment with these agents, most children will have responded if they are going to do so. Continued improvement with longer treatment has been reported. Doses are generally as follows:
- Etanercept: 0.8 mg/kg/wk;
- Infliximab: 6-10 mg/kg/infusion (usually monthly until full response, then lengthened if tolerated to every eight weeks); and
- Adalimumab: 24 mg/m2 every two weeks.
The most common side effects are injection-site or infusion reactions and upper respiratory-tract infections. Etanercept use for as long as eight years has been reported with no increase in serious infections and no reports of lupus, malignancy, demyelinating disorders, or death.
Anakinra
Anti-IL1 blockade appears to be uniquely effective for children with systemic JIA. The adult dose is 100 mg subcutaneously daily, and reports in children have used 1–2.8 mg/kg/day. The main side effect is injection-site reaction, with burning and pain. This decreases in the second month, according to reports. Incidence of infections does not appear to be greater than in placebo populations.
Abatacept
Abatacept (Orencia) is given as an IV infusion monthly at a dose of 10 mg/kg. Infusion reactions are mild and include dizziness, nausea, or headache, with rare reports of hypotension or hypersensitivity. Mild infections were the most common side effect reported, with some reports of severe infections and two reports of suspected TB. Abatacept has been demonstrated to be effective for JIA polyarthritis with 49% of children achieving ACR pedi-30 improvement.
Rituximab
The most common regimen is 750 mg/m2 (1-gram maximum) intravenously twice, two weeks apart. Infusion reactions are usually mild, but there have been reports of serum sickness after the second dose. Side effects may be lessened by pretreatment with benadryl, acetaminophen, and solumedrol.
It may be best to boost tetanus immunity and also immunize for meningococcus and pneumococcus two to three weeks before starting rituximab (even though patients on MTX and prednisone may not respond well to these immunizations). Some clinicians recommend prevention of serious infection with intravenous immunoglobulin 400 mg/month for at least a year post-treatment, because total immunoglobulin levels are not a reliable indicator of adequacy of response to infection or the ability to respond to an antigenic challenge in patients with rheumatologic diseases who are treated with rituximab.
Sulfaslazine and Hydroxychloroquine
These have been reported to improve JIA, but there are no reports of inactive disease or remission. Sulfaslazine may be particularly useful in those with oligoarthritis or those with enthesitis-related disease. With the availability of biologic therapies, these are not used as frequently today.
Systemic Onset JIA
Systemic onset JIA (SJIA) deserves special discussion because it presents unique challenges including establishing a correct diagnosis and treating persistent severe disease and MAS. A correct diagnosis is crucial because prednisone treatment prior to treatment for a childhood malignancy can decrease the response to chemotherapy and, consequently, survival.
Nearly all children with SJIA will need steroid therapy for treatment of the systemic features. If articular features predominate and there are few or no systemic features or MAS (see below), MTX and/or anti-TNFs can be effective. For patients with both arthritis and systemic features/MAS (or if the latter predominate), cyclosporine and anti-IL1 therapy may be needed. Despite aggressive therapy, about 20% of children with SJIA will have disease that does not respond to these treatments. For these children, there are anecdotal reports of response to combination anti-IL1 and anti-TNF therapy, thalidomide, cytoxan, etoposide, and rituximab. With each of these medications, there is tremendous worry about side effects and need for special surveillance for infection, toxicity, and long-term adverse effects.
Macrophage Activation Syndrome
MAS is the result of overactive macrophages. This condition has been reported in many pediatric rheumatic diseases but occurs with greatest frequency in SJIA either at disease onset, with a flare, or during persistently active disease. MAS presents with a wide spectrum of symptoms and laboratory abnormalities that many clinicians feel may be an integral part of SJIA, rather than a separate event.5 Patients with early, mild MAS may have only mild laboratory abnormalities and active systemic disease while others are critically ill in the intensive care unit with persistent rash, persistent high fevers, central nervous system dysfunction, hemorrhagic abnormalities, and cardio-pulmonary-renal failure. Laboratory abnormalities of MAS reveal increasing C-reactive protein (CRP), d-dimer, ferritin, lactate dehydrogenase, aspartate aminotransferase, prothrombin time, and partial thromboplastin time with falling erythrocyte sedimentation rate (ESR), hematocrit, platelets, white blood cell count, and fibrinogen. Mild abnormalities may respond to treatments initiated for the SJIA. However, if abnormalities worsen, then prompt use of high-dose pulse IV solumedrol and IV cyclosporine are important to halt this potentially fatal process.6
Summary
In conclusion, no one combination of medications has been shown to be fully effective in treating JIA. With what is known to date, the most effective approach for many children may be prednisone (and/or intra-articular steroids), MTX, and an anti-TNF agent at the time of diagnosis to rapidly squelch disease. Once remission has been achieved medications can slowly be decreased to a maintenance regimen. Future research will hopefully provide the insights and tools to identify which patients will rapidly achieve remission with lesser therapies.

Dr. Wallace is professor of pediatric rheumatology at Seattle Children’s Hospital and Regional Medical Center and University of Washington School of Medicine. She received her MD from the University of Michigan and completed her pediatrics residency and pediatric rheumatology fellowship at the University of Washington. She was in private practice pediatrics and rheumatology until she joined Children’s Hospital and Regional Medical Center and the University of Washington in 1984.
Dr. Wallace’s main focus is the aggressive treatment of JIA and other pediatric rheumatic diseases. She has published many of the sentinel studies of methotrexate use in JIA treatment and is a principal investigator of a multicenter trial of early aggressive therapy for JIA. She helped develop an international consensus definition for remission of JIA. Her committee positions have included the Pediatric Rheumatology Sub-board for the American Board of Pediatrics and subcommittees of the ACR, and the Advisory Committee of the Pediatric Rheumatology Collaborative Study Group. She is chair of the Childhood Arthritis and Rheumatology Research Alliance.
References
- Wallace CA, Huang B, Bandeira M, et al. Patterns of clinical remission in select categories of juvenile idiopathic arthritis. Arthritis Rheum. 2005;52:3554-3562.
- Wallace CA. The use of methotrexate in childhood rheumatic diseases. Arthritis Rheum.1998;41:381-391.
- Lovell DJ, Reiff A, Jones OY, et al. Long-term safety and efficacy of etanercept in children with polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2006;54:1987-1994.
- Rossi F, Di Dia F, Galipò O, et al. Use of the Sharp and Larsen scoring methods in the assessment of radiographic progression in juvenile idiopathic arthritis. Arthritis Rheum. 2006;55:717-723.
- Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007; 34:1133-1138.
- Ravelli A, De Benedetti F, Viola S, Martini A. Macrophage activation syndrome in systemic juvenile rheumatoid arthritis successfully treated with cyclosporin. J Pediatr.1996;128:275-278.
- Wallace CA, Ruperto N, Giannini EH, et al. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290-2294.