There are two “new” diseases of the late 20th century—AIDS and the antiphospholipid syndrome.
—Miguel Villardel, MD, professor of medicine, Hospital Valle de Hebron, Barcelona
Since its clinical description in 1983, antiphospholipid syndrome (APS or Hughes Syndrome) has become the domain not only of rheumatologists and obstetricians, but of neurologists, cardiologists, psychiatrists, otolaryngologists, and orthopedists. Like lupus, APS embraces all clinical specialities.
Brief History of APS
In 1983, my colleagues and I described in detail a syndrome involving—uniquely—both arterial and venous thrombosis, and including prominent neurological features, including strokes, seizures, memory loss, and chorea. In addition, this syndrome was associated with occasional thrombocytopenia, livedo reticularis, labile hypertension, recurrent miscarriage, and internal organ thrombosis such as Budd-Chiari syndrome.1,2 We recognized that the syndrome was “sufficiently different from typical systemic lupus to warrant separate consideration”—originally calling it the anticardiolipin syndrome.2 Led by the late Aziz Gharavi, MD, and E. Nigel Harris, MPhil, MD, DM, we set up sensitive immunoassays for antiphospholipid antibodies (aPL), changed the name to APS, and introduced the term primary APS for stand-alone cases.2,3
The worldwide interest in this syndrome has led to its recognition in a wide variety of clinical states increasing diagnosis. Indeed, it seems a fairly safe prediction that APS will overtake lupus in prevalence. A rough one-in-five rule seems to apply for the contribution of APS to various clinical conditions—one in five cases of recurrent miscarriage, one in five strokes in people under 45, one in five cases of deep vein thrombosis (DVT). Eventually, this estimate may also pertain to other major illnesses including myocardial infarction in the young, idiopathic teenage epilepsy, and possibly even migraine.
Pathogenesis and Clinical Features
APS is an autoimmune disease in which antibodies directed against phospholipid-protein complexes appear to lead directly to thrombosis. Like all autoimmune diseases APS has a genetic component, and cases of thyroid disease, Sjögren’s syndrome, and lupus are frequently seen in families with APS.
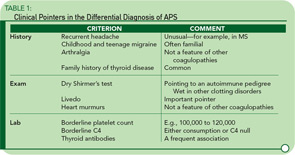
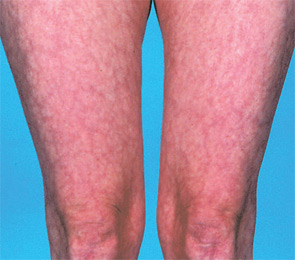
While arterial and venous thromboses are the hallmarks of the syndrome, there are other clinical clues. Headache—particularly migraine—is often an early feature going back to childhood, and often familial. Livedo reticularis is an important physical sign, and a dry Shirmer’s test, suggesting underlying Sjögren’s syndrome, is a clue to the disease’s autoimmune pedigree. Table 1 lists some of the major differences between APS and other pro-thrombotic disorders.
Neurological Considerations
Stroke: The most feared complication of APS is stroke, with up to 20% of strokes in people under 45 possibly having this etiology. In this setting, the MRI can be misleading (e.g., false negatives) and we look forward to the development of more sensitive and available screening techniques. Estimates for the frequency of stroke in patients with APS vary widely, and some studies have been hampered by methodological problems.4
Memory loss: Second only to headache, memory loss is possibly the most common complaint in APS. Despite a number of published psychiatric studies on APS, it is probable that the extent of the problem is still underestimated. Here’s one example from a patient I recently saw: a young woman with APS improved her word-finding score from 15% to 95% after three weeks of subcutaneous heparin. This sort of result is not uncommon in our own clinical experience when formal testing is undertaken.
Multiple sclerosis: In a recent patient survey, 32% of aPL-positive patients in our clinic had had a previous possible diagnosis of MS considered.4 This finding raises important issues. Firstly, differential diagnosis can be difficult. Secondly, we have seen APS patients in whom borderline aPL readings were dismissed as epiphenomena and yet in whom anticoagulation resulted in sustained clinical improvement.
Other neurological: Seizures are a feature of APS.4 Interestingly, in many patients the seizure frequency improves with anticoagulation and worsens with a falling international normalized ratio (INR). Recently, it has been estimated that up to 15% of cases of idiopathic teenage epilepsy may be related to aPL.5 Movement disorders of all descriptions can occur in APS and range from chorea to tics to parkinsonism. Balance problems are particularly common—and, as yet, underreported.
Heart and Lungs
Although the association between aPL and myocardial ischemia is well recognized, there still seems to be a wide range of figures published—between 5% and 15% prevalence of aPL in patients with myocardial infarction (MI), for example. Whatever the figure, the link is of extreme importance in terms of MI prediction and prevention. Sletnes et al., in a study of nearly 600 patients surviving an acute MI, found 13.2% positive for aPL.6 Further, a strong correlation between aPL and the risk of recurrent cardiovascular events in a series of young survivors of MI has been observed.
Although the current message is that routine aPL screening is not indicated in the evaluation of patients with an MI, I disagree. As in the world of obstetrics, a simple, cheap screening test (aPL) could have considerable benefit.
There are two other cardiovascular associations worth noting. First, my colleagues and I have published research on a series of patients with cardiac syndrome X and aPL. Second, increasing evidence points to a role for aPL in the development of accelerated vascular heart disease.7
Pulmonary hypertension: Although the link between APS and pulmonary hypertension has been known since 1983, the extent of the association remains uncertain.2 For example, we have seen a small number of cases of pulmonary hypertension with negative aPL tests at the time of evaluation, but in whom positive aPL and Venereal Disease Research Laboratory slide tests were recorded previously. This situation raises the possibility that in some cases, the thrombotic connection is being missed.
Heart valves: One aspect of APS that separates it from other major clotting disorders is valvular disease. (See Table 1, p. 20.) The use of two-dimensional and Doppler echocardiography has shown a variety of lesions, including thrombotic vegetations, thickening, and stenosis. The mitral valve is by far the most commonly involved, with mitral regurgitation reported in up to one-fourth of all patients.
The pathogenesis of valvular lesions is still uncertain. However, in some cases, a rapid combination of thrombosis and valvular degeneration leads to an early requirement for valve surgery.
Kidneys
The discovery of APS has had at least three important consequences in nephrology. First, it has altered the focus of renal biopsy in lupus to include renal microthrombosis. Second, it has proved an important prognostic (and therapeutic) indicator in renal transplantation. Third, it has revealed the interesting discovery of renal artery stenosis and hypertension associated with aPL.
Marie-Carmen Amigo, MD, and her colleagues at the Instituto Nacional de Cardiologia Ignacio Chavez in Mexico, did much to highlight the important contribution of renal microthrombosis to the pathological picture in lupus, with changes ranging from small occlusive arteriolar thrombotic lesions to severe renal artery hyperplasia and occlusion with renal parenchymal ischemia.8
In renal transplantation, it is clear that patients with APS are at increased risk for post-transplant renal thrombosis and, importantly, that anticoagulant therapy could improve outcome.9 The important experience gained from observations of aPL on the outcome of renal transplantation has led to more routine aPL testing in the fields of liver, heart-lung, and marrow transplant surgery.
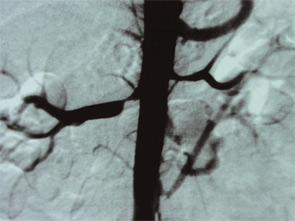
The link between renal artery stenosis and APS has been one of the most interesting and potentially one of the most important. In the 1983 description of APS, I reported “these patients’ blood pressure often fluctuates, apparently correlating with the severity of the livedo, suggesting a possible reno-vascular etiology. However, this group of patients rarely has primary renal disease.”2 Recently, my colleagues (notably Shirish Sangle, MD, and David D’Cruz, MD) have published a series of patients with renal artery stenosis and hypertension. In a cohort of 600 patients with aPL, 173 (29%) had definite hypertension requiring therapy.11 We have now built up a series of APS patients with renal artery stenosis, depicted in Figure 1. The lesions are localized and clearly differ from those of atheroma or fibro-muscular dysplasia.
With full anticoagulation, the hypertension in this group of patients proved easier to control. Interestingly, these focal stenotic arterial lesions are seen elsewhere in the body, for example, in the cerebral circulation and in the celiac and mesenteric arteries.
Other Clinical Features
Gastroenterology: As well as Budd-Chiari syndrome—of which APS is a major cause—more focal hepatic thrombosis is frequent, and abnormal liver function tests are frequent in poorly controlled APS. Idiopathic cirrhosis has also been reported in some patients. Celiac axis arterial stenotic lesions were first fully appreciated in angiographic studies of renal artery stenosis. The classical symptoms of post-prandial mesenteric angina were present in most—though not all—of the patients.
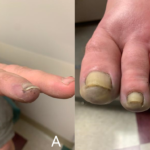
Skin: Livedo reticularis is not only an important marker for APS; it could well be a risk factor in itself. Patients with sero-negative APS—as described below—include some with florid livedo. Thrombosis—particularly DVT and skin vessel thrombosis—leads to skin ulceration. For those who conduct skin ulcer clinics, it is clear that a subset of patients exists in whom chronic skin ulceration could well be improved with anticoagulation.
Ear, nose, and throat: My own practice has seen a sharp increase in the number of patients referred by otolaryngologist colleagues, with APS presenting as acute Ménierè’s and balance problems, as well as the well-documented acute sensory neural hearing loss. Tinnitus is a frequently overlooked complaint and I have previously reported the anecdotal experience of tinnitus in APS improving with anticoagulation.
Blood: Severe thrombocytopenia is fortunately unusual, though borderline platelet counts (e.g., 100,000 to 120,000) are frequent. A possible diagnostic clue is the frequency of pseudothrombocytopenia on automatic ethylenediaminetetraacetic acid blood counts (improving on visual counts). This finding possibly points toward subtle platelet membrane abnormalities.
Orthopedics: My colleagues and I have reported a series of idiopathic metatarsal fractures in APS patients, and case reports of other bone fractures (seemingly unrelated to osteoporosis) have appeared. Perhaps the biggest impact in the world of orthopedics will come from more routine screening for prothrombotic conditions prior to hip or knee surgery.12
Pregnancy and in vitro fertilization: The improvement in pregnancy outcome in aPL-positive women during the past two decades has arguably been the headline story of APS and is reviewed elsewhere.13,14 The role of aPL in the pathogenesis of in vitro fertilization (IVF) implantation failure, among other findings, has led to the use of heparin and aspirin in some IVF regimes, though as yet there is little convincing evidence of benefit.
Sero-negative APS: I assume that all of us see patients with many of the hallmarks of APS, including arterial thrombosis, thrombocytopenia, valve lesions, recurrent pregnancy loss, and so on, in whom aPL tests remain doggedly negative. Explanations for this situation might include wrong diagnosis, a disappearance of antibodies over time, or simply that more aPL-related tests are needed. As a clinician, however, I believe that the concept of sero-negative APS is useful, just as sero-negative RA and sero-negative lupus were decades before.15
Anecdotes for the Clinically Curious
Neurological features of APS almost certainly embrace sleep disturbance. In my clinic, I have an extended family with both APS and autism, which may just be a clinical coincidence. Three of my patients have developed acute anosmia. Peripheral and autonomic nerve problems and cases of reflex dystrophy are also being described. As in diabetes, it may be that ischemia of peripheral nerves renders them more sensitive to external pressure. One of my APS patients with lumbar disc disease and severe symptoms of spinal stenosis and claudication improved once anticoagulation was started. Fatigue, as in lupus, is a frequent and major complaint, and often improves significantly once anticoagulation is started. More difficult to explain, but certainly a frequent observation, is the improvement in the arthralgia (in primary APS) with anticoagulants.
Table 1 (see below) gives a list of features which I believe are of help in diagnosis—certainly useful in the differentiation from other coagulopathies.
Treatment
The indications for aspirin (or clopidogrel), heparin, and warfarin are reviewed in detail elsewhere, and I will restrict this section to three specific comments.
First, the use of a two- to three-week heparin trial in APS patients with severe frequent headache but with normal MRIs may be informative. Although a “soft” diagnostic tool with significant placebo input, in some patients the improvement or even abolition of headache and migraine with a course of self-administered heparin (e.g., Fragmin 10,000 units daily) gives some support to the difficult decision to move to warfarin.
Second, it is important to achieve a therapeutic INR with warfarin, ideally with a self-testing INR machine. There is probably no overall correct INR. Usually, arterial thrombosis appears to require a higher INR than venous. In neurology, however, we frequently see a higher INR requirement (e.g., 3.5–4), with the headaches, TIA, and memory impairment predictably returning when the INR falls below, for example, a precise reading of 3.4. For such patients, the use of self-testing machines is as important to a reasonably normal life as is self-measurement of insulin dose in some diabetics.
Third, the use of immunosuppressives has as yet had little effect on disease manifestations. However, research showing possible direct effects of aPL (e.g., neuronal tissue) and the increasing anecdotal experience of success in APS with new agents such as rituximab may stimulate a reappraisal.
Summary
This review highlights some of the clinical and diagnostic aspects of APS, or Hughes Syndrome. With recognition of the syndrome spreading to all disciplines of medicine, the clinical approach to many diseases is changing. APS is proving a differential diagnosis in diseases such as multiple sclerosis and Alzheimer’s disease and in clinical research it is providing insights into mechanisms of hypertension and accelerated atheroma. In the world of lupus, the recognition of APS has had a profound impact both on diagnosis and on treatment.
Dr. Hughes is head of the London Lupus Centre and London Bridge Hospital.
References
- Hughes GRV. Thrombosis, abortion, cerebral disease and lupus anticoagulant Brit Med J. 1983;287:1088–1089.
- Hughes GRV. Hughes Syndrome The antiphospholipid syndrome. A historical view. Lupus. 1998;7:1–4.
- Harris EN, Gharavi AE, Boey ML, et al. Anticardiolipin antibodies: detection by radio immunoassay and association with thrombosis in SLE. Lancet. 1983;2:1211–1244.
- Hughes GRV. Migraine, memory loss and ‘multiple sclerosis’. Neurological features of the antiphospholipid (Hughes) syndrome. Postgrad Med J. 2003;79:81–83.
- Cimaz R, Meroni PL, Shoenfeld Y. Epilepsy as part of SLE and systemic antiphospholipid syndrome (Hughes Syndrome). Lupus. 2006;15:191–194.
- Sletnes KE, Smith P, Abdelnoor M, et al. Anticardiolipin antibodies after myocardial infarction and their relation to mortality, reinfarction and stroke. Lancet. 1992;339:451–453.
- Hughes GRV, Phillips H.Mycophenolate mofetil and cardiovascular disease. Lupus. 2006;15 (supplement):1–3.
- Amigo MC, Garcia-Torres R. Kidney disease in antiphospholipid syndrome. Chapter in: Hughes Syndrome: antiphospholipid syndrome 2nd Ed. (Ed Khamashta MA). 2006 Springer, London.
- Stone JH, Amend W, Criswell LA. Antiphospholipid antibody syndrome in renal transplantation. Amer J Kidney Dis. 1999;34:1040–1047.
- Sangle S, D’Cruz D, Khamashta MA, Tungekar M, Abbs I, Hughes GRV. Goldblattt’s kidney, Hughes Syndrome and hypertension. Lupus. 2002;11:699–703.
- Sangle S, D’Cruz D, Jan W, et al. Renal artery stenosis in antiphospholipid syndrome and hypertension. Ann Rheum Dis. 2003;62:999–1002.
- Vasoo S, Sangle S, Zain M, Cruz D, Hughes GRV. Orthopaedic manifestations of the antiphospholipid (Hughes) syndrome. Lupus. 2005;14:339–345.
- Derksen RH, Khamashta MA, Branch DW. Management of the obstetric antiphospholipid syndrome. Arthritis Rheum. 2004;50:1028–1039.
- Lockshin MD. Autoimmunity, infertility and assisted reproductive technologies. Lupus. 2004;13:669–672.
- Hughes GRV, Khamashta MA. Sero-negative antiphospholipid syndrome. Ann Rheu Dis. 2003;62:1127.