Extrapolating from rheumatic fever, a number of investigations addressed molecular mimicry as the basis of ReA. These studies sought to operationalize the recent discovery of HLA-B27 as a risk factor not only for ReA but also for ankylosing spondylitis (AS). Approaches to linking infection to disease included demonstration of sequence homology between B27 and arthritogenic pathogens, as well as the characterization of monoclonal antibodies and T-cell clones reacting with both B27 and the putative inciting organism (see Figure 1, p. 24).6,7 Some experimental models suggested that Chlamydia could break tolerance of self-B27.8 However, rigorous proof that autoreactivity is the underlying mechanism in ReA remained difficult to obtain, and ReA remained a charter member of the seronegative spondyloarthropathies.
The report that phlogistic components of Yersinia and Salmonella could be recovered from synovial fluid of patients with ReA suggested that, rather than autoimmunity acting as the operational event in chronic ReA, local persistence of microbial fragments could be the true culprit. The presence of such foreign material was demonstrated by immunofluorescence studies of synovial fluid and synovial tissues.9 These findings shifted the focus of investigators to address the structural basis of arthritogenicity. Thus, certain glycosylation patterns of foreign antigens appeared to correspond with the ability to induce experimental ReA, by virtue of lingering as nonbiodegradable fragments in the joint.10 Gas chromatography–mass spectrometry analysis of organisms could identify such patterns, but this approach has yet to be transferred into clinical practice.
Innate Immunity Weighs In
For all the sophistication of adaptive immunity, with specificity and memory as its pinnacle achievements, it was recognized that the time required to expand the T-cell and B-cell responses in the setting of acute infection could place the host in great peril. The concept of innate immunity as the first line of host defense had been the province of leukocyte biology students, subsequently drosophila scholars. The discovery of toll-like receptors (TLRs) as the quintessential recognition elements of the innate immune system created an intense interest in defining the role of this family of receptors in infectious disease. However, the initial concepts proposed by the late Charles Janeway, MD, and colleagues seemed to have placed some conceptual hurdles in the path of linking TLR to rheumatic diseases.
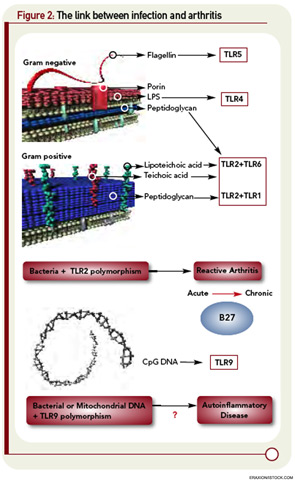
The first of these hurdles related to the apparent nonpolymorphic nature of the TLR, posing a significant challenge to addressing the heterogeneity of susceptibility to ReA in target populations. The second was that self/nonself discrimination by the innate immune system was proposed to be perfect, unlike its forever failing counterpart in adaptive immunity. Both of these concepts have needed significant revision. There are, in fact, significant polymorphisms in the TLR families, and recent studies in epidemic ReA have demonstrated that nonsynonymous single nucleotide polymorphisms in TLR2 confer susceptibility to post-Salmonella ReA (see Figure 2, p. 28).11 The notion that TLRs are specific only for foreign determinants has been replaced with the recognition that mammalian nucleic acids, among other molecules, are ligands for certain TLRs; indeed, this concept has proved to be pivotal in current concepts of lupus pathogenesis.