BACK SURGERY
Long-term Outcome of Herniated Disc Surgery
By David G. Borenstein, MD
Abstract
Study design: A retrospective, follow-up cohort study.
Objective: To evaluate the 25-year (or longer) outcome of discectomy for lumbar disc herniations by validated instruments.
Summary of background data: A comprehensive patient-oriented evaluation should include measurements of pain and disability along with a reliable evaluation of the general health status. There is a paucity of data from validated measuring instruments on the very long-term outcome of lumbar discectomy.
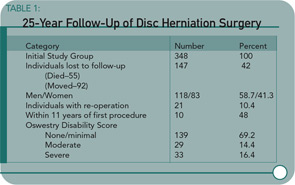
Methods: The authors conducted a follow-up study of 201 patients an average of 27.8 years (range 25-32) after lumbar discectomy. The patient-oriented assessment included a Short Form-36 Health Survey questionnaire, Oswestry Disability Index, Cumulative Illness Rating Scale, and a study specific questionnaire dealing with daily life activities and satisfaction with the surgery.
Results: The Short Form-36 Health Survey physical scales and summary scores were similar to the normative values for healthy subjects and were better than the scores of patients with untreated sciatica with respect to reported pain. The mean Oswestry disability score was 17.5. Satisfaction with surgery was expressed by 181 of 201 patients (90%).
Conclusions: Patients who had undergone lumbar discectomy a minimum of 25 years earlier have a satisfactory self-reported health-related quality of life and less pain than non-surgically treated subjects.
Commentary
An important concern for patients with radiculopathy associated with intervertebral disc herniation involves the better short-term and long-term outcomes associated with surgical discectomy versus medical therapy. Patients need to balance the possibility of rapid resolution of back and leg pain against the potential risks of advanced spinal degeneration caused by surgical intervention. Prior studies have reported a benefit for surgical patients early in the post-operative period. In a prospective study of 126 patients randomized to surgical discectomy versus conservative therapy, the difference in early surgical benefits tends to equalize over a 10-year follow-up period as reported by Weber.1 However, the effects of surgery on clinical symptoms and advancement of spinal degeneration over a longer period than a decade were unknown.
I was reassured by the overall results of this long-term, 25-year follow-up study reported by Mariconda and colleagues demonstrating the satisfaction of surgical patients with their operations as documented by validated outcome measures. A majority of patients undergoing surgery can be pain-free and functional without progressive spinal disorders. I was concerned, however, about several aspects of the study. For example, a significant number of individuals from the initial cohort have been lost to follow-up. Also, a small but significant proportion of study enrollees required additional spinal surgeries and were disabled.
Another of my concerns with the authors’ conclusions regards the unfavorable comparison of normative data of pain score outcomes for medically treated patients reported in the Italian medical literature with the surgical patients in this study. First, the characteristics of these historical medical patients are unknown and the duration of their follow-up is unspecified. Second, while pain scores were lower in surgical patients versus the historical controls, physical function measures were comparable in both groups. Therefore, I would not consider the current study definitive in assessing the relative long-term benefits of medical versus surgical therapy.
Patients with herniated discs are interested in practical outcomes. They are concerned with pain relief, the preservation of function, and limiting future episodes of disc hernation and progressive spinal disease. A recent study by Weinstein et al. reports the equally favorable outcomes of medical and surgical therapy for herniated discs at two years.2 The study by Mariconda et al. demonstrates the potential of those individuals who choose surgery to have a continued good outcome a quarter of a century later.
CANCER
Lymphoma Risk in Patients with Ankylosing Spondylitis
By David G. Borenstein, MD
Abstract
Background: Several inflammatory conditions are associated with an increased risk of lymphoma. The specific features of inflammation that mediate this risk are unknown. There are few studies on whether ankylosing spondylitis (AS) increases the risk of lymphoma. Besides inflammation-lymphoma etiology, information on risk of lymphoma in ankylosing spondylitis is particularly important as a benchmark in the evaluation of, for example, TNF inhibitors.
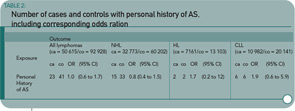
Methods: The association between ankylosing spondylitis and malignant lymphomas overall (and separately for non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, and chronic lymphocytic leukemia) was assessed in a nationwide, population-based, case-control study of 50,615 cases of lymphoma and 92,928 matched controls by using prospectively recorded data on lymphomas from the Swedish Cancer Register (1964–2000) and data on pre-lymphoma hospitalizations for ankylosing spondylitis from the Swedish Inpatient Register (1964–2000). The odds ratios (ORs) associated with pre-lymphoma hospitalization for ankylosing spondylitis were calculated using conditional logistic regression.
Results: Twenty-three (0.05%) patients with lymphoma and 41 (0.05%) controls had a pre-lymphoma hospitalization listing ankylosing spondylitis, relative risk=1.0 (95% confidence interval [CI] 0.6 to 1.7). The number of discharges and the mean latency between ankylosing spondylitis and lymphoma were similar in patients and controls. Analyses restricted to lymphomas diagnosed during the 1990s showed similar results (OR=1.3, 95% CI 0.6 to 2.5, number of exposed cases/controls=14/21).
Conclusion: On average and in the absence of TNF inhibitors, patients hospitalized with ankylosing spondylitis do not show an appreciable increased risk of lymphoma.
Commentary
In a time when there is significant discussion in the medical community and press regarding the risk of lymphoma in patients treated with anti-TNF agents, Askling and colleagues’ article is an important and welcome addition to the current state of knowledge.
To date, there has been little published regarding risks of malignancies in patients with AS or other spondyloarthropathies—with the exception of those who received radiation treatment in past decades. Of the two prior publications found on Medline, the first is from the same authors examining a Swedish population-based cohort prior to the use of anti-TNFs. They reported a standardized incidence ratio of 1.34 (CI 0.93–1.89) for all hematopoietic cancers. The second is an abstract that’s not easily available for review.
There are limitations to this study—particularly with the use of an inpatient discharge registry to identify patients with a diagnosis of AS, which may limit generalizability. Conversely, the patients who require hospitalization may represent a sicker population. In light of the recent studies suggesting that chronic inflammation may be a contributing factor to lymphoma origin in RA, studying a sicker population may be appropriate. Additional potential limitations include the lack of disease severity measurements and the overall small numbers despite using an entire country’s registry. However, these limitations should not overshadow the fact that this is a well designed and executed study that highlights the strengths of an organized data collection system.
The published data suggest that there is not a significant increase in lymphoma risk—certainly not of the magnitude seen with RA. (See Table 2, below.) The distinct disease pathophysiology may contribute to the differences in lymphoma rates. Or perhaps it may be simply the differences in levels of chronic inflammation, which are generally lower in the AS patient.
In summary, this article draws attention to the need estimate lymphoma risk in patients with AS and other spondyloarthropathies prior to the use of anti-TNF agents from other populations. In future studies addressing this knowledge gap, authors should gather information on measures of disease severity, cumulative disease activity, and disease duration in patients to allow us to explore the potential contribution of inflammation in an epidemiological fashion. Similar questions regarding the potential increased risk of lymphoma in the setting of anti-TNF agents are bound to arise, and without background data, the true answer will never be realized or understood.
BONE LOSS
Preventing Fractures in Patients with RA
By Daniel Hal Solomon, MD, MPH
Abstract
Objectives: To examine whether treatment with anti-TNF alpha prevents loss of bone mineral density (BMD) at the spine and hip (generalized) and in the hands (local) of patients with RA, and to study the changes in markers of bone metabolism, including receptor activator of the NFkappaB ligand (RANKL) and osteoprotegerin (OPG), during anti-TNF treatment.
Patients and methods: One hundred two patients with active RA who were treated with infliximab during one year were included in this open cohort study. The BMD of the spine and hip (dual X-ray absorptiometry) and hands dual X-ray radiogrammetry was measured before the start of treatment and after one year. Changes in osteocalcin formation, beta-isomerised carboxy terminal telopeptide of type 1 collagen (beta-CTx, resorption), RANKL, and OPG were determined at 0, 14, 30, and 46 weeks.
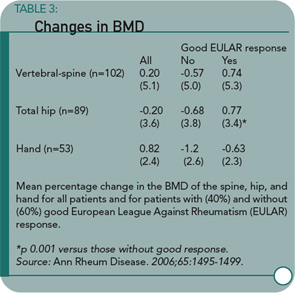
Results: The BMD of the spine and hip was unchanged during treatment with infliximab, whereas BMD of the hand decreased significantly by 0.8% (p<0.01). The BMD of the hip in patients with a good European League Against Rheumatism (EULAR) response showed a favorable change compared with patients not achieving such a response. Serum beta-CTx and RANKL were both considerably decreased compared with baseline at all time points. The decrease in beta-CTx was associated with the decrease in Disease Activity Score of 28 joints (DAS-28) and C reactive protein during the 0-14 weeks interval.
Conclusion: In patients with RA treated with infliximab, spine and hipbone loss is arrested, whereas metacarpal cortical hand bone loss is not stopped. The outcome of the study also supports a relationship between clinical response, in terms of reduced inflammatory activity, and changes in bone loss of the spine, hip, and hands.
The bone abnormalities associated with RA include juxtaarticular osteopenia, focal bone erosion, and diffuse bone loss that extends far beyond the region of the joint. Osteoporosis and the associated fractures appear increased in some groups with RA compared with controls.1 While our understanding of the pathophysiology of bone loss in RA is incomplete, many studies suggest that receptor activator of NFkB ligand (RANKL) and osteoprotegrin (OPG) play important roles in regulating bone metabolism. In turn, TNFa regulates RANKL and OPG, suggesting that the TNFa antagonists may retard not only focal bone erosions but also generalized osteoporosis among patients with RA.
This study by Vis and colleagues builds on several preliminary investigations that suggest a relationship between TNFa antagonist use and improved BMD. In this multicenter study, consecutive patients treated with infliximab for at least one year were included. These patients had all undergone dual energy X-ray absorptiometry (DEXA) measurements at baseline and at follow-up. All patients had DAS-28 scores of at least 3.2 and had previously failed at least two DMARDs. Thirty-four patients dropped out because of an inadequate response or side effects to infliximab; they were not included in the analyses.
The study produced several notable findings. First, the spine and hip BMD was lower at baseline in patients with erosive disease than in those without. Second, there were no significant changes in BMD at the hip or spine over the year follow-up in patients taking infliximab. Third, the soluble RANKL decreased during the follow-up period, whereas OPG remained stable, resulting in a reduced RANKL/OPG ratio. Fourth, patients with a good response to infliximab according to EULAR criteria had a statistically significant improvement in hip BMD compared with those with less than a good response. The same trend was observed with spine and hand BMD, but these were not statistically significant.
Important limitations of the study include the lack of detailed information about glucocorticoid use (just reporting whether or not they were used, not obtaining cumulative dosage data), the substantial dropout because of inadequate response to infliximab, and the lack of a group without infliximab exposure. The authors suggest that infliximab arrested generalized bone loss but this is really impossible to say based on their study design.
The relationship between generalized bone disease and RA is receiving increased attention. It may be possible that treatments for RA, such as TNFa antagonists, not only improve disease activity but also improve BMD. It is also possible that they improve BMD through a positive effect on weight-bearing activity. At this point, I will continue to aggressively screen and treat patients with RA for osteoporosis. Several treatments have been shown to prevent secondary fractures, including bisphosphonates. Calcium and vitamin D should be strongly considered for most patients with RA. A bisphosphonate may be considered in patients with a known minimal trauma fracture, osteoporosis by BMD testing, or chronic glucocorticoid use with osteopenia.