What is not discussed but sorely needed—at least by me—are age- and size-adjusted normal values for muscle enzymes. Although a CK of 199 in a two-year-old may be “normal” by lab standards, it is way out of proportion to muscle mass. These authors address an important issue but the pediatric rheumatology community should take this opportunity to reconsider all of the old criteria as well as the invasive ones.
I would love to know which analytes are most sensitive indicators of myositis (CK, aldolase, and/or markers of endothelial activation such as VWF) and obtain age and body mass normal values. Similarly, age-related norms for muscle strength, using the CMAS (Childhood Myositis Assessment Scale-14), as was done for the earlier version CMAS9, would be useful for evaluating “how weak is weak.” Through the Paediatric Rheumatology European Society and CARRA (Childhood Arthritis and Rheumatology Research Alliance) and their JDM interest sections, the community has the network to do these studies. Recruiting healthy children to donate a teaspoon of blood may be another story.
ARTHRITIS
Continued Debate on TNF Antagonists and Infection
By Daniel H. Solomon, MD, MPH
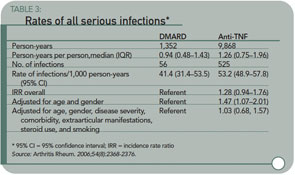
Dixon WG, Watson K, Lunt M, et.al. British Society for Rheumatology Biologics Register Control Centre Consortium. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: Results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54(8):2368-2376.
Abstract
Objective: To determine whether the rate of serious infection is higher in anti–tumor necrosis factor (anti-TNF)-treated RA patients compared with RA patients treated with traditional disease-modifying antirheumatic drugs (DMARDs).
Methods: This was a national prospective observational study of 7,664 anti–TNF-treated and 1,354 DMARD-treated patients with severe RA from the British Society for Rheumatology Biologics Register. All serious infections, stratified by site and organism, were included in the analysis.
Results: Between December 2001 and September 2005, there were 525 serious infections in the anti–TNF-treated cohort and 56 in the comparison cohort (9,868 and 1,352 person-years of follow-up, respectively). The incidence rate ratio (IRR), adjusted for baseline risk, for the anti–TNF-treated cohort compared with the comparison cohort was 1.03 (95% confidence interval 0.68–1.57). However, the frequency of serious skin and soft tissue infections was increased in anti–TNF-treated patients, with an adjusted IRR of 4.28 (95% confidence interval 1.06–17.17). There was no difference in infection risk between the three main anti-TNF drugs. Nineteen serious bacterial intracellular infections occurred, exclusively in patients in the anti–TNF-treated cohort.
Conclusion: In patients with active RA, anti-TNF therapy was not associated with increased risk of overall serious infection compared with DMARD treatment, after adjustment for baseline risk. In contrast, the rate of serious skin and soft tissue infections was increased, suggesting an important physiologic role of TNF in host defense in the skin and soft tissues beyond that in other tissues.
Commentary
Just when you thought that the last word had been written on the risk of infection associated with TNF antagonists, another important observational study suggests that these drugs may not be associated with an increased risk in typical practice. The study from the British Society for Rheumatology Biologics Register joins several other large analyses from cohorts showing no increased risk for infection associated with TNF antagonists.