Ankylosing Spondylitis
Gene Association Hints at Potential Ankylosing Spondylitis Treatment
By Maripat Corr, MD
Abstract
We have genotyped 14,436 nonsynonymous single nucleotide polymorphisms (nsSNPs) and 897 major histocompatibility complex (MHC) tag SNPs from 1,000 independent cases of ankylosing spondylitis (AS), autoimmune thyroid disease (AITD), multiple sclerosis (MS), and breast cancer (BC). Comparing these data against a common control dataset derived from 1,500 randomly selected healthy British individuals, we report initial association and independent replication in a North American sample of two new loci related to AS—ARTS1 and IL23R—and confirmation of the previously reported association of AITD with TSHR and FCRL3. These findings, enabled in part by increased statistical power resulting from the expansion of the control reference group to include individuals from the other disease groups, highlight notable new possibilities for autoimmune regulation and suggest that IL23R may be a common susceptibility factor for the major “seronegative” diseases.
Commentary
The new platforms for high-throughput genetic screening are rapidly expanding our knowledge of susceptibility loci for complex diseases. Investigators for the Wellcome Trust Case Control Consortium and the Australo-Anglo-American Spondylitis Consortium recently reported their findings from a large, genome-wide scan of four diseases. The strongest associations observed in the study were between SNPs in the major histocompatibility (MHC) encoding region and the three autoimmune diseases studied: AS, AITD, and MS. The genome-wide scan also identified and validated two new genes—ARTS1 (type 1 tumor necrosis factor receptor shedding aminopeptidase regulator) and IL23R (interleukin 23 receptor)—to be associated with AS.
Functionally, ARTS1 and IL23R represent interesting biological candidates for association with AS. The ARTS1 (also known as ERAAP, or ERAP1) gene encodes a type II integral transmembrane aminopeptidase with intra- and extracellular functions. In the endoplasmic reticulum, ARTS1 is involved in processing peptides to the optimal length for MHC class I presentation.1,2 This activity is intriguing because of the strong association of AS with the class I allele HLA-B27. A genetic association with the peptide loading process suggests a mechanistic link to HLA-B27 in developing disease. Alternatively, ARTS1 cleaves cell surface receptors for pro-inflammatory cytokines like IL-1, IL-6, and tumor necrosis factor (TNF), reducing the number of surface receptors and releasing soluble receptor antagonists.3-5 Potentially, a variant of ARTS1 with reduced ability to cleave surface receptors would prolong the interval that cells could receive signals from inflammatory cytokines.
Susceptibility to Crohn’s disease and psoriasis have also been reported to be associated with polymorphisms in the IL23R gene.6,7 This shared association suggests that there may be a common mechanism in the pathogenesis of seronegative spondyloarthropathies that lends itself to treatment. IL-23 shares the p40 protein subunit with IL-12 and plays a critical role in the generation of effector memory T cells and IL-17-producing T cells. These IL-17-producing T cells are critical in sustaining organ specific inflammation in several different mouse models of autoimmune disease. Targeting IL23R would theoretically also reduce IL-17 production. Therapeutic trials assessing the efficacy of IL12/23 inhibitors in Crohn’s disease are underway.8,9 The genetic link between AS and Crohn’s disease implies that clinical success using the IL12/23 inhibitors in Crohn’s disease may translate therapeutically to AS by targeting a common mechanism.
References
- Chang SC, Momburg F, Bhutani N, Goldberg AG. The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc Natl Acad Sci USA. 2005;102:17107-17112.
- Saveanu L, Carroll O, Lindo V, et al. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat Immunol. 2005;6:689-697.
- Cui X, Rouhani FN, Hawari F, Levine SJ. Shedding of the type II IL-1 decoy receptor requires a multifunctional aminopeptidase, aminopeptidase regulator of TNF receptor type 1 shedding. J Immunol. 2003;171:6814-6819.
- Cui X, Rouhani FN, Hawari F, Levine SJ. An aminopeptidase, ARTS-1, is required for interleukin-6 receptor shedding. J Biol Chem. 2003;278:28677-28685.
- Cui X, Hawari F, Alsaaty S, et al. Identification of ARTS-1 as a novel TNFR1-binding protein that promotes TNFR1 ectodomain shedding. J Clin Invest. 2002;110:515-526.
- Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461-1463.
- Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273-290.
- Billich, A. Drug evaluation: Apilimod, an oral IL-12/IL-23 inhibitor for the treatment of autoimmune diseases and common variable immunodeficiency. IDrugs. 2007;10:53-59.
- Burakoff R, Barish CF, Riff D, et al. A phase 1/2A trial of STA 5326, an oral interleukin-12/23 inhibitor, in patients with active moderate to severe Crohn’s disease. Inflamm Bowel Dis. 2006;12:558-565.
Fractures
New Tool for Predicting Hip Fracture Risk
By Daniel Hal Solomon, MD, MPH
Abstract
Context: The 329,000 hip fractures that annually occur in the United States are associated with high morbidity, mortality, and cost. Identification of those at high risk is a step toward prevention.
Objective: To develop an algorithm to predict the five-year risk of hip fracture in postmenopausal women.
Design, setting, and participants: A total of 93,676 women who participated in the observational component of the Women’s Health Initiative (WHI), a multiethnic longitudinal study, were used to develop a predictive algorithm based on commonly available clinical features. Selected factors that predicted hip fracture were then validated by 68,132 women who participated in the clinical trial. The model was tested in a subset of 10,750 women who had undergone dual-energy X-ray absorptiometry (DXA) scans for bone mass density assessment.
Main outcome measure: The prediction of centrally adjudicated hip fracture, measured by the area under the receiver operator characteristic (ROC) curves.
Results: During a mean (SD) follow-up of 7.6 (1.7) years, 1,132 hip fractures were identified among women participating in the observational study (annualized rate, 0.16%), whereas during a mean follow-up of 8.0 (1.7) years, 791 hip fractures occurred among women participating in the clinical trial (annualized rate, 0.14%). Eleven factors predicted hip fracture within five years: age, self-reported health, weight, height, race/ethnicity, self-reported physical activity, history of fracture after age 54 years, parental hip fracture, current smoking, current corticosteroid use, and treated diabetes. ROC curves showed that the algorithm had an area under the curve of 80% (95% confidence interval [CI], 0.77%–0.82%) when tested in the cohort of different women who were in the clinical trial. A simplified point score was developed for the probability of hip fracture. ROC curves comparing DXA-scan prediction based on a 10% subset of the cohort and the algorithm among those who participated the clinical trial were similar, with an area under the curve of 79% (95% CI, 73%–85%) versus 71% (95% CI, 66%–76%).
Conclusion: This algorithm, based on 11 clinical factors, may be useful to predict the five-year risk of hip fracture among postmenopausal women of various ethnic backgrounds. Further studies are needed to assess the clinical implication of the algorithm in general and specifically to identify treatment benefits.
Commentary
Hip fractures cause substantial morbidity, mortality, and related costs. While many pharmacologic agents have been developed for treating osteoporosis, an unacceptably high proportion of patients do not receive treatment until after a fracture, and many receive no treatment even after sustaining a fracture. Timely bone mineral density (BMD) testing can help identify at-risk individuals. However, most fractures occur in persons who do not have osteoporosis by t-score thresholds, pointing out the importance of non–BMD-related risk factors. Robbins and colleagues used the very large WHI datasets to develop and test a clinical prediction rule for hip fractures in postmenopausal women.
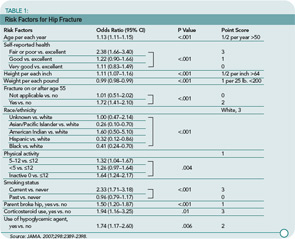
These investigators used data from nearly 100,000 women participating in the observational component of the WHI to identify eleven risk factors that were strong and significant predictors of hip fractures. These are listed in Table 1 (p. 22) and include older age, worse self-reported health, height above 64 inches, weight less than 200 pounds, fracture after 54 years of age, white race, physical inactivity, current smoking, parental history of hip fracture, corticosteroid use, and use of a hypoglycemic agent. Many of these risk factors have been well described in prior literature. These factors were then combined using a scoring system (available in a calculator form at http://hipcalculator.fhcrc.org) and compared with the five-year fracture rates.
The clinical prediction rule was found to be a good predictor of hip fractures within five years with areas under the ROC curve (AUC) of approximately 0.80. The performance of the clinical prediction rule varied by the predicted five-year risk of hip fracture. For example, at low five-year predicted risk value (i.e., 0.1%), the rule was very sensitive but less specific. At higher five-year predicted risk values (i.e., 1%), the rule was very specific but less sensitive. The predictive ability of the rule was tested against BMD in a subset of women and found to have a slightly lower AUC, 0.71 versus 0.79. As expected, combining the clinical prediction rule with BMD information gave the best predictive information.
This study was well conducted and pursues an important clinical area. There are many non-BMD risk factors that should be assessed when considering how to counsel women (and men) about their risk of a future fracture. This study informs us about some important factors and gives precise information about combining these factors for a precise estimate of risk. The clinical prediction rule and its calculation can easily be accessed and used via the Internet. However, because most drug treatment trials have not used such a risk calculator to define included populations, it is unclear how treatment will affect the calculated risks. I look forward to the day when clinical prediction rules are combined with treatment trials to better guide our therapeutic decision-making.