Ibuprofen Best for Acute Traumatic Musculoskeletal Injuries
By Kathleen A. Haines, MD
Clark E, Plint AC, Correll R, Gaboury I, Passi B. A randomized, controlled trial of acetaminophen, ibuprofen, and codeine for acute pain relief in children with musculoskeletal trauma. Pediatrics. 2007; 119(3):460-467.
Abstract
Objective: The authors’ goal was to determine which of three analgesics—acetaminophen, ibuprofen, or codeine—given as a single dose, provides the most efficacious analgesia for children presenting to the emergency department with pain from acute musculoskeletal injuries.
Patients and methods: Children six to 17 years old with pain from a musculoskeletal injury (to extremities, neck, and back) that occurred in the preceding 48 hours before presentation in the emergency department were randomly assigned to receive orally 15 mg/kg acetaminophen, 10 mg/kg ibuprofen, or 1 mg/kg codeine. Children, parents, and the research assistants were blinded to group assignment. The primary outcome was change in pain from baseline to 60 minutes after treatment with study medication as measured by using a visual analog scale.
I was sorely disappointed that, as the authors state, at best 50% of children achieved adequate analgesia with the highest recommended dose of ibuprofen.
Results: A total of 336 patients were randomly assigned, and 300 were included in the analysis of the primary outcome (100 in the acetaminophen group, 100 in the ibuprofen group, and 100 in the codeine group). Study groups were similar in age, gender, final diagnosis, previous analgesic given, and baseline pain score. Patients in the ibuprofen group had a significantly greater improvement in pain score (mean decrease: 24 mm) than those in the codeine (mean decrease: 11 mm) and acetaminophen (mean decrease: 12 mm) groups at 60 minutes. In addition, at 60 minutes more patients in the ibuprofen group achieved adequate analgesia (as defined by a visual analog scale <30 mm) than the other two groups. There was no significant difference between patients in the codeine and acetaminophen groups in the change in pain score at any time period or in the number of patients achieving adequate analgesia.
Conclusions: For the treatment of acute traumatic musculoskeletal injuries, ibuprofen provides the best analgesia among the three study medications.
Commentary
Pediatric rheumatologists deal with pain. More often than not, pain is the symptom that brings children to our offices. If we make a diagnosis of an inflammatory arthritis, pain control often appears to be relatively straightforward, as we prescribe NSAIDs, DMARDs, and possibly corticosteroids to diminish inflammation and the resultant pain. However, the majority of children who present with pain complaints do not have juvenile inflammatory arthritis or other arthritis. Rather, they have patello-femoral syndrome, hypermobility, or the dreaded “growing pains”—those mysterious wandering leg pains that have the family up half the night and the child completely recovered by daybreak. Which analgesic agent works best for musculoskeletal pain in children is a question of interest to the pediatric rheumatologist.
The authors of the above study have investigated whether acetaminophen, ibuprofen, or codeine provides the most effective pain relief—as determined by rapidity of onset and by amount of relief—in children age six to 17 with acute musculoskeletal pain. They recruited 336 children with soft-tissue injury or closed fracture and randomly assigned them to receive one of the above three agents at the maximal recommended dose of each agent. The analgesics were formulated as a purple, grape-flavored liquid given by a nurse not involved in the study to maintain blinding, as the volumes were not identical among the drugs. Pain scores using a 100 mm visual analog scale (VAS) were recorded at 0, 30, 60, and 120 minutes after drug administration. (The use of the VAS in children older than six has been validated.) A 15-mm change in pain score was considered to be clinically significant and pain rated at <30 mm was considered adequate pain control. Both of these endpoints fall within acceptable ranges in several studies of pain measure in children.
The baseline pain scores in each group were not significantly different, ranging between 51 and 57 mm VAS. However, only the group receiving ibuprofen had a clinically significant diminution of pain after 60 minutes (-27 mm, p<0.001). In addition, 52% of patients receiving ibuprofen achieved adequate pain relief as measured by a VAS of <30 mm. Patients receiving acetaminophen did not have clinically significant pain relief until 60 min (-17 mm) and those receiving codeine reported pain relief at 120 min (-17 mm). Only 36% and 40% of the acetaminophen and codeine groups respectively achieved adequate pain control. In patients with a high degree of pain at baseline (VAS >30 mm), ibuprofen provided the most rapid relief, achieving significance by 30 minutes; codeine was equally effective at 120 minutes. Of interest, patients with fractures received the most benefit from ibuprofen. Diminution of pain in patients with soft-tissue injuries did not achieve significance in any group. Side effects were equivalent.
One of the more common myths of adulthood is that children do not suffer from pain without serious illness. Yet cross-sectional studies of the prevalence of pain in children have demonstrated between 20% and 40% of children have pain at any one time. Looking retrospectively, 80% of school-age children will report having had pain at some time in a three-month period. Hence, knowing how to treat pain in children is clearly important. Upon encountering this study, I assumed it would help me overcome my reluctance to use narcotic analgesics in treating pain. However, I was startled to see ibuprofen outperforming codeine. On second look, I was sorely disappointed that, as the authors state, at best 50% of children achieved adequate analgesia with the highest recommended dose of ibuprofen.
I am not sure how to translate this study in children with acute pain to our patients with various pain syndromes. Growing pains usually respond very nicely to NSAIDs, perhaps reflecting the acuity of this problem. Patients with hypermobility and pain and those with patello-femoral syndrome often have more chronic pain. Do these patients parallel those in the cohort with soft tissue injury, who were more resistant to pain relief, or the group with fracture, who received reasonable pain relief—or are they different still? My take-home message for hypermobile/patello-femoral pain: try pushing ibuprofen higher than my usual 10 mg/kg and add on a narcotic analgesic (at least for short periods) and get those kids to physical therapy. I think I will save acetaminophen for treatment of low-grade fever.
The Role of DKK-1 in RA
By Maripat Corr, MD
Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13(2):156-163.
Abstract
Degenerative and inflammatory joint diseases lead to destruction of the joint architecture. Whereas degenerative osteoarthritis results in the formation of new bone, rheumatoid arthritis leads to bone resorption. The molecular basis of these different patterns of joint disease is unknown. By inhibiting Dickkopf-1 (DKK-1), a regulatory molecule of the wingless (Wnt) pathway, we were able to reverse the bone-destructive pattern of a mouse model of rheumatoid arthritis to the bone-forming pattern of osteoarthritis. In this way, no overall bone erosion resulted, although bony nodules, so-called osteophytes, did form. We identified tumor necrosis factor–a (TNF-a) as a key inducer of DKK-1 in the mouse inflammatory arthritis model and in human rheumatoid arthritis. These results suggest that the Wnt pathway is a key regulator of joint remodeling.
Commentary
Inflammatory arthritis is coupled with structural changes in the underlying bone. These bony changes can be either erosive, as seen in rheumatoid arthritis (RA), or proliferative, as exemplified by ankylosing spondylitis. Hence, soluble regulators of bone remodeling have been a topic of intensive investigative interest. Recently, the Wnt signaling pathway has been identified as a key pathway in maintaining adult bone mass and bone turnover.
Wnt proteins are extracellular ligands which bind to the G-protein-coupled seven-transmembrane domain frizzled receptors and the low-density lipoprotein receptor-related protein 5 and 6 (LRP5/6) coreceptors. Receptor ligation triggers a cascade of events that stabilizes beta-catenin, and enables its nuclear translocation and subsequent activity as a transcriptional cofactor.1 Gain of function polymorphisms in the LRP 5 coreceptor results in elevated bone mass and bony proliferation, and loss of function variants result in the osteoporosis pseudoglioma syndrome.2,3 The DKK family of soluble Wnt antagonists binds to LRP and another receptor called Kremen. (See Figure 1) Diarra et al. have identified one of these molecules, DKK-1, as a key modulator of bone remodeling disorders. In addition its potential as a therapeutic target, serum levels of his protein might be a surrigate biomarker for disease-associated bone remodeling.
To examine the role of DKK-1 in inflammatory arthritis, Diarra and colleagues used a monoclonal antibody to mouse DKK-1 in three separate models of murine arthritis. Although treatment with an anti-TNF antibody diminished paw swelling, anti–DKK-1 administration had no effect on paw size. However, anti–DKK-1 protected against bone erosion and there was an increased formation of bony nodules (osteophytes) at the joint margins in the anti–DKK-1 treated animals. Fewer erosions also correlated with fewer osteoclasts seen in the anti–DKK-1 treated animals. An additional effect of anti–DKK-1 treatment on inhibiting bone resorption was the associated increase in osteoprotegerin (OPG) expression. OPG is a soluble antagonist for the receptor activator of NF-kB ligand (RANKL), a critical factor for osteoclast differentiation. The effect of anti–DKK-1 on resorption appeared to be secondarily mediated through OPG. After experimental reduction in articular OPG expression, the osteoclasts re-emerged in anti–DKK-1–treated animals, supporting this hypothesis. In previous reports, DKK-1 had been found to suppress osteoblast differentiation. Hence, DKK-1 might toggle the balance between bone repair and resorption
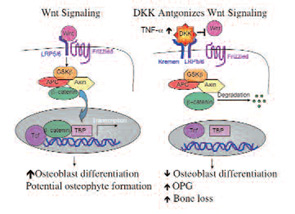
Although the source of systemic DKK-1 remains unclear, inflamed synovium from RA patients was shown to express DKK-1 locally in the joint. In addition, fibroblast-like synoviocytes were induced to express DKK-1 by exposure to TNF-a in culture. As a correlate, serum levels of DKK-1 declined progressively during six weeks in RA patients who were started on anti-TNF therapy. In addition, serum levels of DKK-1 were proportional to their disease activity score (DAS) 28, whereas ankylosing spondylitis patients had low baseline levels of circulating DKK-1.
This elegant series of experiments suggests that DKK-1 or other Wnt signaling modulators might prove to be useful therapeutic targets for therapies to reduce bone erosion in RA. However, in addition to attenuating bone erosion the experimental mice had aberrant bony proliferation, suggesting that osteophyte formation may be a potential side effect that would require monitoring. In addition, the Wnt pathway regulates multiple cellular functions including proliferation and differentiation. Wnt signaling antagonists have been reported as key molecules in tumor suppression, angioneogensis, and cardiac disease amongst other potential comorbidity issues. These initial findings by Diarra et al. are very promising in a potential new target to abrogate bone erosion, but further investigating into the effects on other organ systems is warranted.
References
- Glass DA, 2nd, Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology. 2007, Mar 29; (Epub ahead of print).
- Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513-1521.
- Gong Y, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513-523.