Cadherin-11 May Be a Therapeutic Target in RA
By Mary Corr, MD
Lee DM, Kiener HP, Agarwal SK, et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315(5814):1006-1010.
Abstract
The normal synovium forms a membrane at the edges of joints and provides lubrication and nutrients for cartilage. In RA, the synovium is the site of inflammation, and it participates in an organized tissue response that damages cartilage and bone. We identified cadherin-11 as essential for development of the synovium. Cadherin-11–deficient mice have a hypoplastic synovial lining, display a disorganized synovial reaction to inflammation, and are resistant to inflammatory arthritis. Cadherin-11 therapeutics prevent and reduce arthritis in mouse models. Thus, synovial cadherin-11 determines the behavior of synovial cells in their proinflammatory and destructive tissue response in inflammatory arthritis.
Commentary
RA is a debilitating disease marked by bone and cartilage destruction. In the rheumatoid synovium, the fibroblast cells aggressively proliferate and invade the other joint structures. Indeed, these fibroblast-like synoviocytes develop genetic and phenotypic features similar to malignant cells, including expression of proto-oncogenes and loss of contact growth inhibition. Although these cells in the pannus locally invade, they never metastasize. Most of the current therapies for RA are directed toward other components of the immune system. There has been limited investigation into therapies that are directed against the aggressive behavior of the synovial fibroblasts. By targeting cadherin-11, an essential adhesion molecule for synovial development and mature architecture, Lee and colleagues are paving the way for another approach in our therapeutic armamentarium.
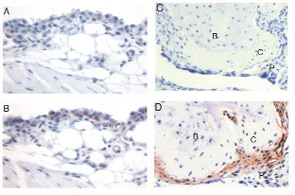
Source: Science. 2007;315(5814):1007.
In a normal diarthrodial joint, the synovial lining is composed of synoviocytes that form an intact layer through cell-to-cell contacts with intervening matrix spaces. Cadherins are a family of adhesion molecules that bind to other cadherin molecules on neighboring cells. Cadherin-11 has a key role in mediating fibroblast-like synoviocyte intercellular adhesion and synovial tissue organization. (See Figure 1) Mice that are genetically deficient in cadherin-11 have a hypoplastic synovial lining. When challenged with arthritogenic antibodies, cadherin-11–deficient mice developed less paw swelling than normal mice and the synovial tissue appeared to lack the usual dense organization on histological examination. In this experiment, fibroblasts in the local microenvironment appeared necessary for the full function of other cell types in the inflammatory process.
The relative resistance of the cadherin-11–null mice to induced arthritis indicates that this molecule might be a therapeutic target. Using complementary approaches with a cadherin-11–specific monoclonal antibody and a cadherin-11–Fc fusion protein, the investigators showed that both reagents suppress the induction of disease. However, the treatment of established disease was less effective than prevention of acute arthritis in mice. Migration and invasion of synoviocytes are clearly affected by the function of cadherin-11. Arthritic cadherin-null mice develop bone erosions; however, synovial attachment and migration of synoviocytes into cartilage were markedly diminished. An opposing picture was previously described in mice deficient in the receptor activator of nuclear factor kappa B ligand (RANKL); these mice developed severe cartilage damage without bone erosion in the same model of induced arthritis. Cartilage damage in the RANKL-deficient mice reflected direct synovial pannus activity rather than a secondary effect from deterioration of the underlying bone. In the cadherin-null mice, there is relative preservation of the cartilage in the face of bone destruction and a dysfunctional synovium.
The precise mechanism for the improvement in anti-cadherin–treated mice has yet to be determined. Do these treatment protocols result in synoviocyte apoptosis, migration, or senescence? The long-term consequences of anti-cadherin therapy to joint organization and integrity also need to be addressed. Disrupting the usual cohesion of hyperproliferative pannus might be a successful strategy in secondarily undermining the ability of immune cells to deliver localized proinflammatory signals. In addition, a treatment that targets the cohesiveness of the synovial architecture might prove to be a useful adjunctive therapy to immunosuppressive therapies without further increasing the risk of severe infections. A synergistic agent with minimal additional risk would be highly beneficial in our therapeutic arsenal.
When Treating Uveitis, All Anti-TNFs May Not Be Equal
By Michael M. Ward, MD
Guignard S, Gossec L, Salliot C, et al. Efficacy of tumor necrosis factor blockers in reducing uveitis flares in patients with spondyloarthropathy: a retrospective study. Ann Rheum Dis. 2006;65:1631-1634.
Abstract
Objective: To evaluate the efficacy of anti–tumor necrosis factor (TNF) treatments (given for rheumatological manifestations) in reducing uveitis flares in patients with spondylarthropathy in daily practice.
Methods: A retrospective observational study of all patients with spondylarthropathy with at least one uveitis flare treated with anti-TNF in one center (Dec. 1997–Dec. 2004). The number of uveitis flares per 100 patient-years was compared before and during anti-TNF treatment; each patient was his or her own control. The relative risk (RR) and the number needed to treat (NNT) were calculated.
Results: Forty-six patients with spondylarthropathy treated with anti-TNF drugs had at least one uveitis flare (33 treated with anti-TNF antibodies—infliximab or adalimumab—and 13 with soluble TNF receptor—etanercept). The mean age at first symptoms was 26 years, 71% were men. Patients were followed for 15.2 years (mean) prior to anti-TNF versus 1.2 years during anti-TNF treatment. The number of uveitis flares per 100 patient-years before and during anti-TNF were, respectively: for all anti-TNF treatments: 51.8 versus 21.4 (p=0.03), RR=2.4, NNT=3 (95% confidence interval [CI] 2 to 5); for soluble TNF receptor: 54.6 versus 58.5 (p=0.92), RR=0.9; and for anti-TNF antibodies: 50.6 v 6.8 (p=0.001), RR=7.4, NNT=2 (95% CI 2 to 5).
Conclusion: Anti-TNF treatments were efficacious in decreasing the number of uveitis flares in patients with spondylarthropathy. Anti-TNF antibodies decreased the rate of uveitis flares, whereas soluble TNF receptor did not seem to decrease this rate. These results could have consequences for the choice of anti-TNF treatment in certain patients.
Commentary
Acute anterior uveitis occurs in up to 40% of patients with spondyloarthritis. Recurrent episodes are common and uveitis may be the disease’s most distressing feature for some patients. Although most flares can be treated effectively with local therapy, for patients with frequent flares, it is important to determine if systemic therapy can prevent recurrences or reduce flare frequency. Sulfasalazine and methotrexate have been used with some success to prevent recurrences of uveitis. The growing use of anti–TNF-alpha medications to treat spondyloarthritis has generated interest in determining if these medications can prevent uveitis flares, and whether effectiveness differs among anti-TNF medications.
This observational study compared rates of uveitis episodes (calculated per 100 patient-years) before and after the start of treatment with etanercept or anti-TNF antibodies (infliximab or adalimumab). The study was limited to patients with spondyloarthritis who had at least one uveitis episode (although the episode may have occurred after the start of treatment), who were starting their first course of anti-TNF medication, and who had observation on treatment for at least one week. The mean length of observation on treatment was 1.2 years. The study was retrospective; all episodes of uveitis occurred and treatment was started before the study began. The number of uveitis episodes per patient, extending back to illness onset, was determined from clinical records and patient report.
The 13 patients treated with etanercept had similar rates of uveitis episodes before treatment and after the start of treatment. (See Table 1) For the 25 patients treated with infliximab and eight patients treated with adalimumab, the rate was substantially lower after the start of treatment. The number of uveitis flares per patient before treatment was higher among those treated with anti-TNF antibodies than those treated with etanercept, but the length of observation before treatment was also longer in the anti-TNF antibody group.

Although few patients were studied, these results are interesting and potentially clinically useful. This study adds to the growing literature on the ability of anti-TNF medications to prevent recurrences of uveitis and supports this literature in suggesting that infliximab may be more effective than etanercept in this regard. There are few data from other studies on whether adalimumab can prevent uveitis. However, this study did not have an untreated control arm, and therefore we do not know how these patients fared compared to patients who were not treated. The accuracy of the methods used in this study to document episodes of uveitis was not reported, and recall by patients of episodes that occurred in the distant past might have been poor, but would be expected to be similar in all patient groups. Concomitant treatment with other disease-modifying medications might have contributed to differences between treatment groups, but similar proportions received these medications, and no patients received sulfasalazine. The mechanisms potentially responsible for the differential effectiveness of etanercept and anti-TNF antibodies have not been explored, although parallels have been drawn to Crohn’s disease treatment.
This study suggests infliximab or adalimumab might be preferred treatments for patients with spondyloarthritis who have indications for anti-TNF treatment for musculoskeletal symptoms and frequent uveitis episodes. Firmer evidence of a differential effect among anti-TNF medications might come from studies of biological registries, although these sources might not include data on pre-treatment uveitis episodes. While this study is timely, it should not distract us from considering conventional disease-modifying medications. Future clinical trials should include a placebo arm as well as sulfasalazine and methotrexate arms.
