SNOWMASS VILLAGE, COLO.—Data from three recent trials in systemic sclerosis (scleroderma) provide information on a number of important issues related to screening and treatment. First presented at the 2018 ACR/ARHP Annual Meeting, the phase 2/3 trials assessed the safety and efficacy of targeted agents to treat patients with systemic sclerosis.1-3
In a follow-up presentation at the 2019 ACR Winter Rheumatology Symposium, Dinesh Khanna, MD, MSc, Frederick G.L. Huetwell Professor of Rheumatology, professor of internal medicine in the Division of Rheumatology, at the University of Michigan, Ann Arbor, and director of the University of Michigan Scleroderma Program, discussed key points highlighted by all three trials (see Table 1, below).
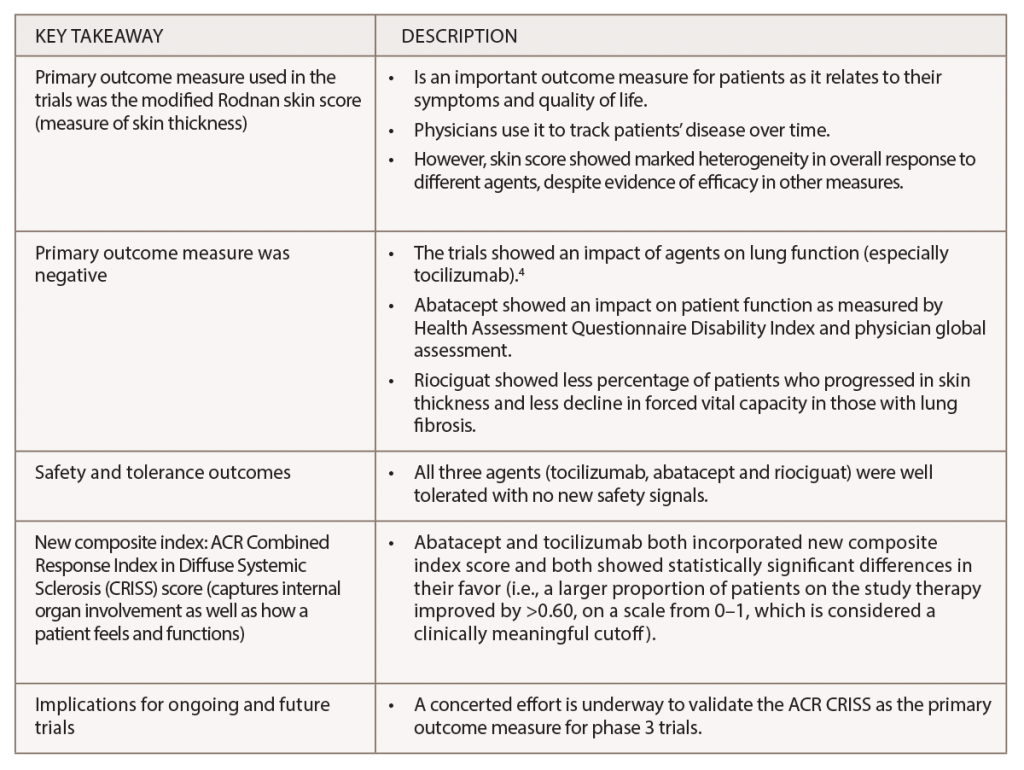
(click for larger image) Table 1: Key Findings from Recent Trials on Targeted Therapy for Systemic Sclerosis
Lessons Learned
Dr. Khanna also discussed lessons learned from the trials about the potential for targeted scleroderma therapies.
One lesson is the importance of understanding the natural history of systemic sclerosis and recognizing that most complications involving the internal organs occur early in the disease. Organ involvement typically occurs two to five years after the first non-Raynaud’s phenomenon symptom and may include lung, heart and renal problems.
Because internal organ involvement in most patients is irreversible, Dr. Khanna emphasized the importance of screening patients to ensure early detection and treatment. Internal organ involvement not detected and treated early can be fatal. Cardiopulmonary involvement (i.e., lung fibrosis and pulmonary arterial hypertension) is the leading cause of mortality in these patients.
Dr. Khanna also stressed the need to closely follow patients in whom the initial workup is negative to ensure early detection of internal organ involvement should it present.
One controversial question raised in the session was how aggressively to screen for internal organ involvement given the risks of tests for lung fibrosis (chest computed tomography with the risk of radiation) and pulmonary arterial hypertension (right heart catheterization is invasive and carries rare risks). For Dr. Khanna, the benefits of aggressive early screening outweigh the risks. “Our center aggressively screens these patients as we believe that early screening and diagnosis lead to better outcome measures,” he said.
Data support this belief. Dr. Khanna discussed a study showing improved survival in patients in whom pulmonary arterial hypertension is detected at an early stage, compared with historical cohorts.5 However, he also emphasized that using an echocardiogram for screening for pulmonary hypertension is associated with a high rate of false negatives and consideration should be given to combining different non-invasive measures for screening.6
Finally, the trials provided information on which targeted therapies show promise in treating specific complications. Phase 2 and phase 3 data now show that tocilizumab benefits lung function in patients even with a negative modified Rodnan skin score, and phase 2 data show patient function is improved with abatacept.
An important discussion focused on data related to autologous stem cell transplantation and its viability as a treatment option for systemic sclerosis. Dr. Khanna presented recent trial data from the SCOT trial on the long-term efficacy of myeloablative autologous transplantation for severe scleroderma.7 The study found a significant survival benefit at 11 years in patients treated with autologous transplantation compared with those treated with cyclophosphamide (88% vs. 53%; P=0.01).7
A primary takeaway, says Dr. Khanna, is the importance of early referral for a transplant consultation before organ failure to allow for shared decision making with patients about treatment choice.
Mary Beth Nierengarten is a freelance medical journalist based in Minneapolis.
References
- Khanna D, Spino C, Bush E, et al. Abatacept vs. placebo in early diffuse cutaneous systemic sclerosis—results of a phase 2 investigator initiated, double-blind, placebo-controlled, multicenter, randomized controlled trial study (abstract 900). Arthritis Rheumatol. 2018;70(suppl 10).
- Khanna D, Lin CJF, Kuwana M, et al. Efficacy and safety of tocilizumab for the treatment of systemic sclerosis: Results from a phase 3 randomized controlled trial (abstract 898). Arthritis Rheumatol. 2018;70(suppl 10).
- Distler O, Allanore Y, Denton CP, et al. Riociguat in patients with early diffuse cutaneous systemic sclerosis: A randomized, double-blind, placebo-controlled phase IIb study (RISE-SSc) (abstract 903). Arthritis Rheumatol. 2018;70(suppl 10).
- Khanna D, Denton CP, Jahreis A, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): A phase 2, randomised, controlled trial. Lancet. 2016 Jun 25;387(10038):2630–2640.
- Kolstad KD, Li S, Steen V, et al. Long-term outcomes in systemic sclerosis-associated pulmonary arterial hypertension from the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma Registry (PHAROS). Chest. 2018 Oct;154(4):862–871.
- Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019 Jan 24;53(1).
- Sullivan K, Pinckney A, Goldmuntz E, et al. Myeloablative autologous hematopoietic stem cell transplantation for severe scleroderma: Long-term outcomes 6–11 years after entry on a randomized study comparing transplantation and cyclophosphamide (abstract 1820). Arthritis Rheumatol. 2018;70(suppl 10).


