Case Report

Pixsooz / shutterstock.com
A 78-year-old man with pulmonary fibrosis was referred to a rheumatologist on Jan. 19, 2018, because of a positive rheumatoid factor test. His joint examination was normal. Although reassured he did not have RA, he asked to be monitored by both a rheumatologist and a pulmonologist.
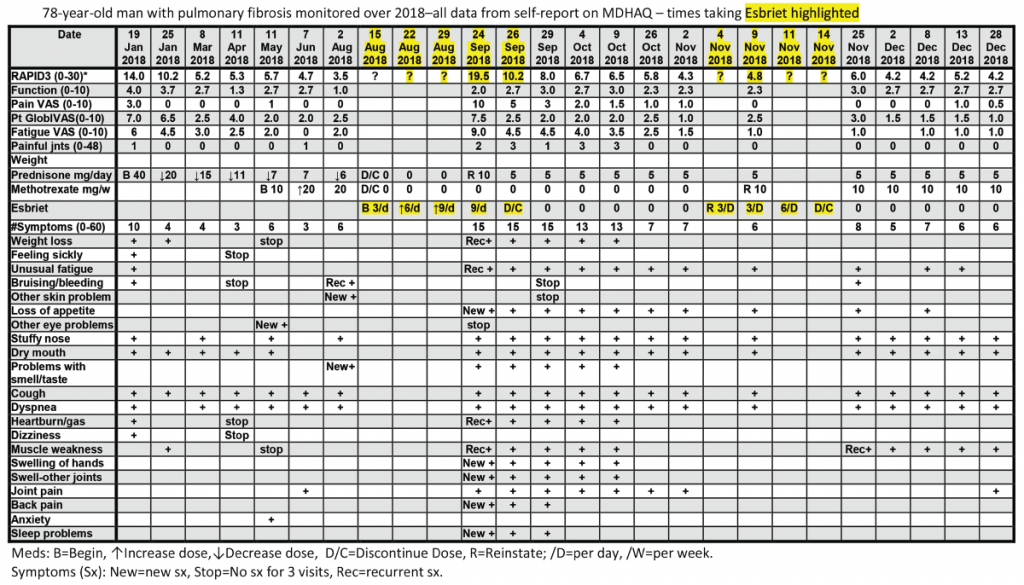
All patients with all diagnoses seen in the rheumatology division at Rush University Medical Center complete an MDHAQ at all routine visits. At the patient’s first visit, his RAPID3 score was 14/30 (high severity = 12–30), his fatigue score on the 0–10 VNS was 7/10, and he reported 10/60 symptoms (see Figure 2, below).6 He was treated with low-dose prednisone and methotrexate, experiencing substantial clinical improvement over the next six months, which was documented on the MDHAQ. On Aug. 2, the RAPID3 score was 3.5/30 (low severity = 3.1–6.0), his fatigue score was 2/10, and he reported 6/60 symptoms (see Figure 2, below), although he continued to require low-level oxygen.
On Aug. 15, his pulmonologist discontinued low-dose prednisone and methotrexate and prescribed 267 mg pills of pirfenidone (Esbriet), an anti-fibrotic agent, escalated over three weeks: three pills the first week, six the second and nine the third (see Figure 2, below).16 His next rheumatology appointment was scheduled for October.
On Sept. 24, the patient telephoned the rheumatologist to report many new problems, although his pulmonary status was mostly unchanged—continuing to require oxygen. The rheumatologist sent the patient an electronic MDHAQ for completion at home to characterize his clinical status quantitatively for comparison with the Aug. 2 report. His new RAPID3 score was 19.5/30 vs. 3.5/30 on Aug. 2, his fatigue score was 9/10 vs. 2/10, and he reported 15/60 vs. 6/60 symptoms (see Figure 2). Seven of the nine new symptoms (not reported on Aug. 2) were among 16 adverse events listed for pirfenidone on the manufacturer’s website (see Figure 3). These included weight loss, anorexia, unusual fatigue, abdominal pain, indigestion, heartburn and insomnia (see Figure 2).
The rheumatologist advised discontinuation of pirfenidone, reinstatement of 10 mg/day of prednisone and remote electronic MDHAQ monitoring two and five days later, with a plan for a face-to-face visit if no improvement was apparent. Five days later, his RAPID3 had improved from 19.5/30 to 8/30 and his fatigue score improved from 9/10 to 4/10 (see Figure 2).
Weekly remote electronic MDHAQ monitoring was instituted, which documented clinical improvement according to RAPID3 and resolution of most pirfenidone-related symptoms over the next 10 weeks (see Figure 2).
A brief retrial of pirfenidone was followed by an increase of the RAPID3 score to 6.0 and was discontinued in a timely manner (see Figure 2, opposite). On Dec. 28 (after methotrexate was reinstated), the patient’s RAPID3 was 4.2/30, his fatigue score was 2.0/10, and he reported 6/60 symptoms.