“[The EU has] had over 10 years of monitoring for any difference between biosimilars and originators, and they haven’t found anything to raise a red flag. They’ve been at the forefront,” says Stanley Cohen, MD, clinical professor, Department of Internal Medicine, University of Texas Southwestern Medical Center and in private practice, Rheumatology Associates, Dallas. “In most of the countries, the health departments are promoting the use of biosimilars as initiating therapy and haven’t determined any issues in terms of switching therapies.”
Safety & Efficacy
Both the report and the overall experience among European physicians indicate no specific safety or efficacy concerns for biosimilars compared with their reference products, says Jonathan Kay, MD, professor of medicine, Timothy S. and Elaine L. Peterson Chair in Rheumatology, and director of clinical research, Division of Rheumatology, University of Massachusetts Medical School, Worcester.
“If a biosimilar is truly biosimilar—and the FDA has strict requirements to demonstrate that—it should have the same safety profile as the parent compound,” says Ellen Gravallese, MD, chief, Division of Rheumatology, professor of Medicine, and Myles J. McDonough Chair in Rheumatology, University of Massachusetts Medical School. Dr. Gravallese is also the ACR secretary.
Efficacy is another aspect on which biosimilars and their reference products seem to match closely. “Comparative effectiveness studies have shown that biosimilars are equivalent to their reference products. When I have spoken with rheumatologists from around the world, no one has mentioned lesser efficacy of a biosimilar,” Dr. Kay says.
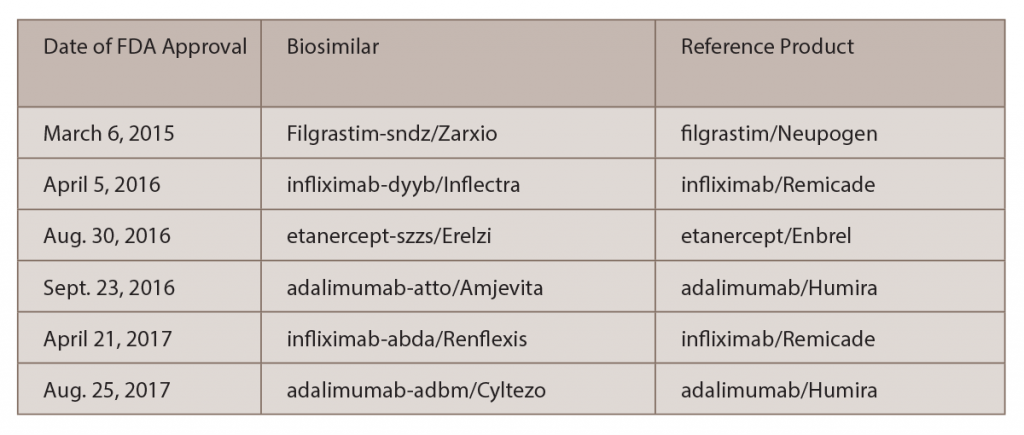
The only mentions of lesser efficacy of a biosimilar that he has seen were in several posters from Turkey that were presented at the 2016 ACR/ARHP Annual Meeting and at the 2017 European League Against Rheumatism (EULAR) Annual Meeting, which suggested that Celltrion’s biosimilar, infliximab-dyyb (Inflectra), was less effective than Remicade. Of note, the analyses reported in these posters were supported by the maker of Remicade.
“Despite the positive experience regarding safety and efficacy of biosimilars elsewhere, it is not unexpected that rheumatologists in the U.S. may have questions about biosimilars,” says Dr. Kay. “Because they haven’t had a lot of experience with biosimilars, they remain uncertain about their safety and efficacy,” he says.
There is confusion among physicians surrounding biosimilars and biomimics, and that can stir up more questions. Although biomimics are replicas of the reference product, they have not gone through the extensive regulatory review process to which biosimilars have been subjected, Dr. Kay adds. “Once a biosimilar has been reviewed and approved by regulators in a highly regulated area, one can be confident that it meets standards of efficacy and equivalence to its reference product,” he says. In contrast, some biomimics do not even have the same amino acid sequence as the reference products they intend to copy.
Interchangeability
Two major areas of interest with regard to biosimilars both in Europe and the U.S. are interchangeability and extrapolation of indications. With interchangeability, when a biologic is prescribed, the patient may actually end up getting a biosimilar. The question is, who can or should make that decision on interchangeability?