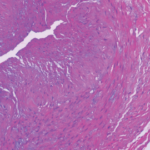
Temporal arteritis was first described by Sir Jonathan Hutchinson in 1890 in an elderly retired gentleman’s servant who developed red, painful streaks on his temples and was found to have bilaterally swollen temporal arteries with feeble pulses.1 Sir Hutchinson disputed the suggestion that the red streaks were caused by the man’s hat and, instead, called his observation, albeit without histology, “an unquestionable example of an arteritis, which spread along the affected vessels, causing swelling of the external coats and adjacent cellular tissue which resulted very quickly in occlusion of the vessels.”
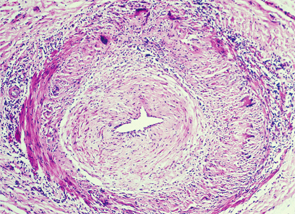
Temporal arteritis is a form of the medium to large vessel arteritis named giant cell arteritis.
Pathology
More than a century has passed since this initial observation, but the sequence of events precipitating the arteritis remains unclear.
Temporal arteritis is now recognized to be a form of the medium- to large-vessel arteritis named giant cell arteritis (GCA) because of characteristic giant cells seen on histology. In addition to branches of the external carotid artery, GCA can affect many other vascular territories, including the ophthalmic artery, causing vision loss in 10–15% of patients; the vertebrobasilar arteries, causing ischemic stroke; the aorta, causing dissection or aneurysm formation; and the subclavian arteries, causing claudication.2,3 The temporal artery biopsy typically demonstrates mononuclear inflammatory infiltrates in the vessel wall, with fragmentation of the elastic lamina. The lumen is often occluded from intimal hyperplasia. Giant cells, when found, are found at the intima-media border.4
There may be an association between VZV & GCA rather than causation.
GCA Trigger
The pathologic features, however, don’t provide an etiology. In the May 12, 2015, issue of Neurology, Don Gilden, MD, professor of neurology at the University of Colorado in Aurora, and colleagues published provocative evidence that varicella zoster virus (VZV) may trigger GCA.5 They examined 82 formalin-fixed biopsy specimens of histologically confirmed GCA along with 13 cadaveric temporal artery controls. Strikingly, immunohistochemical analysis of the arterial wall for VZV gE antigen (produced in infected cells) was positive in 74% of the GCA cases compared with only one positive finding in the control arteries. As is characteristic of GCA pathology, there were “skip areas” where VZV was absent. GCA pathology in 89% of cases tended to be adjacent to regions containing the VZV antigen. In almost half of the cases, the VZV antigen was found in the adventitia of the arterial wall.
The authors confirmed the immunohistochemical findings using other techniques, as well. VZV DNA was detected using polymerase chain reaction (PCR) in 40% of the lesions that were positive using immunohistochemistry. Electron microscopy on a single specimen showed enveloped viral particles within the vessel wall.
The current publication is certainly not the first suggestion that VZV or other infections might be important in the pathogenesis of GCA. In fact, as the authors acknowledge, at least seven prior studies have searched for VZV in arteries affected by GCA.5 Many of these studies were negative. For example, in 2014, Ami Bhatt, MD, and colleagues analyzed 12 formalin-fixed temporal artery biopsy specimens with GCA for microbes using comprehensive DNA sequencing compared to five controls.6 They did not find pathogenic viruses, including VZV.
In 2004, Rodriguez-Pla and colleagues used PCR to search for VZV DNA in 50 temporal artery biopsies showing histologic features of GCA.7 None were positive.
On the other hand, some smaller studies have suggested an association between VZV and GCA. For example, in a 2001 study of 35 specimens of GCA, 26% showed evidence of VZV using PCR compared with none in controls.8
Sampling Error?
The question remains whether the current study, which is larger than any of the previous investigations, definitively advances our understanding of GCA or is yet another inconclusive finding in the long-running debate about GCA and VZV.
One of the strengths of this study is how comprehensively the histological sections were analyzed. The authors not only studied sections showing GCA pathology but also analyzed the skip areas for the presence of VZV. As it turned out, VZV-positive regions tended to be adjacent to GCA pathology rather than being part of it. Secondly, the authors performed PCR selectively on regions that were positive for VZV using immunohistochemistry techniques. Because VZV-positive regions have intervening gaps, it’s possible the mixture of positive and negative VZV PCR publications reflects the substantial sampling error when trying to detect VZV. The one electron micrograph presented that shows enveloped viral particles is hard to ignore.
However, the study is cross-sectional, and we can say that there may be an association between VZV and GCA rather than causation. Also, the controls were from autopsy and obtained from different conditions than the temporal arteritis samples.
If we are to believe the authors’ argument that VZV is causative of GCA secondary to VZV reactivation in the cranial ganglia followed by axonal spread to the arterial adventitia, how do we reconcile the apparent brisk therapeutic response of GCA to high-dose corticosteroids? Although it’s possible the clinical symptoms are caused primarily by an inflammatory reaction to a self-limited viral reactivation, one would expect that treatment with steroids would ultimately lead to more disseminated infection. Examples of steroid-refractory GCA exist, but in the majority of cases, treatment with steroids over months leads to control of symptoms rather than deterioration.
Polymyalgia rheumatica (PMR) also accompanies about 50% of cases of GCA.2 If VZV alone were to account for GCA, it would likely follow that the much more prevalent steroid-responsive condition PMR might be related to VZV as well. The study does not provide clinical data on the patients about preceding symptoms.
Questions Remain
The current study raises important questions about our understanding of GCA. Therapeutically, a randomized clinical trial assessing the value of adding anti-viral treatment to corticosteroids accompanied by proof of VZV treatment, such as by histology, will help answer some of the outstanding questions.
Shamik Bhattacharyya, MD, MS, is a neurologist in the Division of Neurological Infections and Inflammatory Disorders at Brigham and Women’s Hospital, Harvard Medical School, Boston.
References
- Hutchinson J. Diseases of the arteries. Arch Surg. 1890;1:323–331.
- Weyand CM, Goronzy JJ. Clinical practice. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med. 2014 Oct 23;371(1):50–57.
- Borchers AT, Gershwin ME. Giant cell arteritis: A review of classification, pathophysiology, geoepidemiology and treatment. Autoimmun Rev. 2012 May;11(6–7):A544–A554. doi:10.1016/j.autrev.2012.01.003.
- Lie JT. Illustrated histopathologic classification criteria for selected vasculitis syndromes. American College of Rheumatology Subcommittee on Classification of Vasculitis. Arthritis Rheum. 1990 Aug;33(8):1074–1087.
- Gilden D, White T, Khmeleva N, et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology. 2015 May 12;84(19):1948–1955.
- Bhatt AS, Manzo VE, Pedamallu CS, et al. In search of a candidate pathogen for giant cell arteritis: Sequencing-based characterization of the giant cell arteritis microbiome. Arthritis Rheumatol. 2014 Jul;66(7):1939–1944.
- Rodriguez-Pla A, Bosch-Gil JA, Echevarria-Mayo JE, et al. No detection of parvovirus B19 or herpesvirus DNA in giant cell arteritis. J Clin Virol. 2004 Sep;31(1):11–15.
- Mitchell BM, Font RL. Detection of varicella zoster virus DNA in some patients with giant cell arteritis. Invest Ophthalmol Vis Sci. 2001 Oct;42(11):2572–2577.