SAN DIEGO—Over the past few years, a better understanding of the pathogenesis of small- and large-vessel vasculitis has emerged from a clearer picture of the role of the adaptive and innate immune system on disease development, as well a better understanding of the genetic and environmental elements that create conditions conducive for disease development.
Recent advances in the pathogenesis of small- and large-vessel vasculitis were the focus of a session at the 2013 ACR/ARHP Annual Meeting during a session titled, “Novel Insight Into the Pathogenesis of Systemic Vasculitis.” [Editor’s Note: This session was recorded and is available via ACR SessionSelect at www.rheumatology.org.]
New Disease Model
Cornelia M. Weyand, MD, PhD, chief of the division of immunology and rheumatology and professor of medicine at Stanford School of Medicine in Stanford, Calif., focused on giant-cell arteritis (GCA) when presenting a new disease model of large-vessel vasculitis that includes two different pathways involved in the pathogenesis of disease. Chronic inflammation ends up as a “cytokine soup,” she said, but emphasized the importance of identifying the initial event that caused the inflammation to potentially better target treatment.
Although Dr. Weyand said that multiple gaps in knowledge still exist in understanding this pathogenesis, a major clue to a better understanding is the fact that age is the strongest risk factor. She reviewed the aging immune system, focusing on two pathways of pathogenesis of GCA based on clusters of cytokines found in the tissue lesions of patients with GCA: 1) a cluster of interleukin (IL) 12, interferon (INF) gamma, and IL-2; and 2) a cluster of IL-6 and IL-17. Based on this finding, she said a new disease model of large vessel vasculitis proposes that there are two disease-relevant T-cell lineages driving vasculitis: IL-17–producing Th17 cells and INF gamma–producing TH1 cells.
Of these two T-cell lineages, the INF gamma–producing cells (Th1) comprise 20%–30% of the cells in the blood of patients with GCA. This is 10 times as many cells as the Th17, which only comprise 2%–3% of cells. “The Th17 pathway may help light the fire,” Dr. Weyand said, “but does very little in maintaining disease over time.”
The importance of this for treatment is highlighted by the fact that IL 17–producing cells (Th17) are affected by corticosteroid treatment, but that INF gamma–producing cells (Th1) are not.
Summarizing her findings, Dr. Weyand described this new disease model as the triple-hit model:
- The vascular wall plays a role by sending and receiving signals.
- The cytokines IL-6 and IL-17 drive an immune pathway that is easily controlled by corticosteroid therapy and is possibly dispensable for vasculitis.
- The cytokines IL-12 and INF gamma drive an immune pathway that is indispensible for vasculitis and relatively resistant to steroids.
Rula A. Hajj-Ali, MD, a physician in the department of rheumatic and immunologic diseases at the Cleveland Clinic in Cleveland, Ohio, who moderated the session, emphasized that one of the challenges remaining is how to meet the diversity of pathogenesis with multiple therapies, but without overtreatment.
“Another important issue for the clinicians,” she said, “is our ability to quantify disease burden and disease activity with noninvasive testing and identification upstream of pathogenic events.”
For Dr. Weyand, the new disease model demands a rethinking of the approach to assessment and treatment of large-vessel vasculitis, particularly as this condition occurs primarily in older patients. “Short and long term management of the disease has to take into account the age of the patient population,” she said, emphasizing the need to assess the risks of immunosuppression in elderly patients. “The majority of patients with GCA have a normal life expectancy, “she said. “Can long-term immunosuppression really improve the outcome, and can we do long-term immunosuppression with acceptable risk for side effects in an elderly patient population?”
Pathogenesis of Small-Vessel Vasculitis
Ronald Falk, MD, Allan Brewster Distinguished Professor of Medicine at the University of North Carolina in Chapel Hill, focused on three common clinical questions he hears from practicing physicians and patients with immune diseases:
- What is the cause of disease?
- What are the factors involved in disease pathogenesis?
- What causes a relapse or sustains remission?
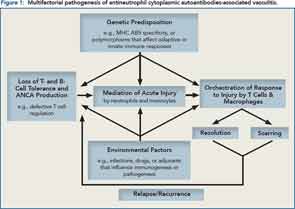
Using data from a genome-wide study published in the New England Journal of Medicine, he discussed findings that suggest the pathogenesis of antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis has a genetic component.1 In particular, the study shows that sets of genes associated with ANCA-associated vasculitis are not clustered according to the disease itself, but rather according to the type of ANCA (i.e., proteinase 3 [PR3] ANCA-associated vasculitis or myeloperoxidase [MPO] ANCA-associated vasculitis) suggesting two distinct autoimmune syndromes.
Along with genetic factors involved in the pathogenesis of disease, he also emphasized the influence of environmental factors that remain to be discovered. All of these factors play a role in why some patients relapse and others sustain a remission. He summarized the pathogenesis of ANCA vasculitis by citing an algorithm he and his colleagues have developed (see Figure 1).
Key Message to Clinicians
For practicing rheumatologists who see patients with small- and large-vessel vasculitis, a key message from this session, according to Dr. Hajj-Ali, is the crucial need to identify the pathogenic pathways in crafting therapeutic modalities. She emphasized the need to identify the triggers that initiate the inflammatory response, and the need for clinicians to recognize that the signature of chronic inflammation may not reflect the initial trigger response.
“The pathogenesis of vasculitides includes multiple factors,” she said. “Genetic predisposition is very important, as well as environmental factors. We also should not neglect the role of the microenvironment where inflammation starts, which sets the stage for a suitable battle field.”
Mary Beth Nierengarten is a freelance medical journalist based in St. Paul, Minn.
