 Over the past decade, a number of pilot studies have provided proof of concept for the use of vagus nerve stimulation (VNS) to treat rheumatic conditions. The studies represent an expansion of this treatment approach into rheumatology, building on years of scientific inquiry into the mechanisms of VNS on disease states that led to preclinical and clinical work showing its safety and efficacy to treat a range of conditions, including epilepsy, Parkinson’s disease, bradycardia and, more recently, depression.
Over the past decade, a number of pilot studies have provided proof of concept for the use of vagus nerve stimulation (VNS) to treat rheumatic conditions. The studies represent an expansion of this treatment approach into rheumatology, building on years of scientific inquiry into the mechanisms of VNS on disease states that led to preclinical and clinical work showing its safety and efficacy to treat a range of conditions, including epilepsy, Parkinson’s disease, bradycardia and, more recently, depression.
For rheumatology, a specialty rooted in the pharmacologic treatment of disease, using electricity to target the inflammatory processes underlying rheumatic diseases represents a novel approach that, if proved effective, could offer needed help to the many patients for whom current medications don’t work sufficiently or are too difficult to tolerate.
“This is the brand-new field of neuromodulation for the treatment of autoimmune disease,” says David Chernoff, MD, chief medical officer of SetPoint Medical, Valencia, Calif., the company leading the research on VNS for the treatment of rheumatoid arthritis. Citing current successful treatments in other fields, such as spinal stimulators for refractory pain, deep brain stimulation for movement disorders, VNS for drug-refractory epilepsy and implantable defibrillators for cardiac issues, Dr. Chernoff hopes clinical studies will show this treatment approach is both safe and effective for rheumatic conditions.

Dr. Chernoff
But much work is needed to establish a place for VNS in rheumatology. Proof-of-concept studies have paved the way for the first randomized controlled trials, but these are just getting underway. Many questions will need answering beyond the safety and efficacy of the treatment approach, such as for whom VNS is indicated, whether to use it as a standalone treatment or as adjuvant to other treatments and, if used with other treatments, which ones (e.g., biologics, Janus kinase [JAK] inhibitors). Such issues as cost and accessibility will loom larger once the evidence is in.
To date, the most advanced evidence for VNS in patients with rheumatoid arthritis (RA) is with the use of an implanted device to stimulate the vagus nerve, with smaller, pilot studies examining the effects of external stimulation of the vagus nerve. Going forward, but premature for this article, will be discussions on the pros and cons of each type of stimulation device.
Evidence to Date
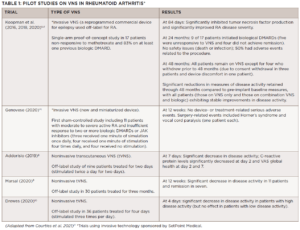
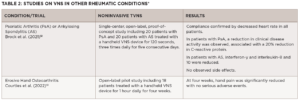
The first pilot studies on the use of VNS in rheumatology focused on RA (see Table 1), with more recent studies looking at its use in primary Sjögren’s disease, psoriatic arthritis/ankylosing spondylitis and osteoarthritis (see Table 2).
The 2020 study by Genovese et al., published in Lancet Rheumatology, is the only trial to date to include a sham arm and, therefore, provides the highest quality of evidence on the safety and efficacy of VNS for RA.1 Noting that the aim of the trial was to assess the safety of implanting the device, which was demonstrated, Dr. Chernoff underscores that the study also showed disease activity was reduced in some of the patients who had failed all available pharmacologic treatments for RA.
Given the results, SetPoint is collaborating with the U.S. Food & Drug Administration in conducting a trial designed to gain approval for VNS for drug-refractory RA. The RESET-RA trial is a multicenter, placebo-controlled, blinded study with a sham arm. It will include 215 patients with refractory RA who will be implanted with the VNS device; the device will initially be activated in half the patients, and assessments of disease activity will be made. At 12 weeks, the device will be activated in all patients and they will be followed for several years to assess short-term and long-term efficacy. All patients in the study previously failed at least one biologic or JAK inhibitor, and all will remain on methotrexate throughout the study.
“If this approach proves effective in patients with drug-refractory RA, it certainly would provide an alternative treatment option for people who have failed successive drugs,” says Dr. Chernoff, noting, however, that he views VNS as complementary to pharmacologic treatments for RA.
“We have to acknowledge that although biologics and targeted synthetic DMARDs [disease-modifying antirheumatic drugs] have been game changers, we have reached a point with all of these drugs that they have significant liabilities,” he says. “We’re trying to provide a viable alternative treatment option.”
Another randomized study currently underway in France will help determine the safety and efficacy of VNS for erosive hand osteoarthritis. The multicenter ESTIVAL trial includes 145 patients with symptomatic and inflammatory disease randomized to an active and sham transcutaneous vagus nerve stimulation (tVNS) treatment group.2 According to the study’s senior investigator, Jeremie Sellam, MD, a rheumatologist at the Saint-Antoine Hospital, Paris, results of the trial are expected later this year.
Looking Forward

Dr. Staats
Peter Staats, MD, chief medical officer, National Spine & Pain Centers, Frederick, Md., whose work has long focused on neuromodulation, underscores the work that lays ahead before VNS becomes a part of rheumatologic care.
“This is one of the most promising areas of development today,” he says. “The power of electricity can’t be overstated and history has shown that it is one of the most powerful tools that we have in managing disease, and we’re going to find the same thing for rheumatologic diseases.”
Dr. Staats, who is also a cofounder and chief medical officer of Basking Ridge, N.J.-based electroCore, a company focused on developing noninvasive VNS technologies to treat disease, underscores that the basic science and mechanisms of action of the vagus nerve on inflammation and other processes are now well understood, but the questions going forward will be whether VNS is effective in specific diseases, such as rheumatologic diseases.
Along with data from randomized trials, continued investigation into the anti-inflammatory mechanisms of action of VNS is providing ongoing insights that may help individualize treatment.

Dr. Olofsson
“It is very important to understand the details of the neurophysiology, molecular mechanisms and immunological effects of peripheral nerve activation in distinct contexts,” says Peder S. Olofsson, MD, PhD, principal researcher, Center for Bioelectronic Medicine, MedTechLabs, Karolinska Institute, Stockholm, and professor, Institute of Bioelectronic Medicine, Feinstein Institutes for Medical Research, Northwell Health, Manhasset, N.Y. He says an improved understanding of these processes may help us better select patients who can benefit from VNS, improve prediction of the effects and potential side effects of VNS, and improve capabilities of monitoring treatment effects and efficacy.
In one study, Dr. Olofsson et al. reported findings shedding light on how nerve signals regulate resolution of inflammation.3 They found that activation of the vagus nerve shortened the time to resolution of inflammation by significantly increasing levels of key molecules (specialized pro-resolving mediators [SPMs]) that promote processes that actively resolve inflammation.
Dr. Olofsson says this finding can be useful in multiple ways, such as measuring how a particular treatment like nerve stimulation changes the levels of SPMs. “This may help improve our understanding of how the disease develops and heals in inflammatory diseases, such as rheumatoid arthritis, and how nerves are involved in these processes,” he says.
Identifying enzymes, mediators and receptors regulated by signals in the vagus nerve can also help improve the understanding of, and the ability to predict, the therapeutic effects of stimulating the vagus nerve. “Since the details of the underlying biology may differ between patients with rheumatoid arthritis, therapeutic interventions may differ in their efficacy depending on whether they directly address the underlying problem,” says Dr. Olofsson.

Dr. Kameneva
Further guidance on individualizing VNS treatment may come from a more detailed understanding of changes in brain activity caused by stimulation of the vagus nerve. A study by investigators from Australia reported on the simultaneous use of magnetoencephalography with tVNS to gain insight into regions of the brain most influenced by stimulating the vagus nerve.4 “We have shown that varying the stimulation frequency can lead to a difference in brain response, with the brain also responding in different anatomical regions depending on the frequency,” says senior author of the study, Tatiana Kameneva, PhD, senior lecturer, School of Science, Computing and Engineering Technologies, Swinburne University of Technology, Hawthorn, Australia.
“This may lead to the development of customized therapeutic approaches for the targeted treatment of different conditions, possibly to rheumatologic diseases.”
Mary Beth Nierengarten is a freelance medical journalist based in Minneapolis.
References
- Genovese MC, Gaylis NB, Sikes D, et al. Safety and efficacy of neurostimulation with a miniaturized vagus nerve stimulation device in patients with multidrug-refractory rheumatoid arthritis: A two-stage multicentre, randomized pilot study. Lancet Rheumatol. 2020 Sep;2(9):e527–e538.
- Courties A, Deprouw C, Rousseau A, et al. Transcutaneous vagus nerve stimulation in erosive hand osteoarthritis: Protocol for the randomized, double-blind, sham-controlled ESTIVAL trial. BMJ Open. 2022 Mar 22;12(3):e56169.
- Caravaca AS, Gallina AL, Tarnawski L, et al. Vagus nerve stimulation promotes resolution of inflammation by a mechanism that involves Alox15 and requires the α7nAChR subunit. Proc Natl Acad Sci U S A. 2022 May 31;119(22):e2023285119.
- Keatch C, Lambert E, Woods W, Kameneva T. Measuring brain response to transcutaneous vagus nerve stimulation (tVNS) using simultaneous magnetoencephalography (MEG). J Neural Eng. 2022 Apr 13:19(2):026038.
- Koopman FA, Chavan SS, Miljko S, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016 Jul 19;113(29):8284–8289.
- Koopman FA, Musters A, Backer M, et al. SATO240 Vagus nerve stimulation in patients with rheumatoid arthritis: Two-year safety and efficacy [abstract]. Annals of the Rheumatic Dis. 2018;77(suppl 2):981–982.
- Koopman FA, Musters A, Backer M, et al. AB1318-HPR Vagus nerve stimulation in patients with rheumatoid arthritis: 48 month safety and efficacy [abstract]. Annals of the Rheumatic Dis. 2020;79(suppl 1): 1948–1949.
- Addorisio ME, Imperato GH, de Vos AF, et al. Investigational treatment of rheumatoid arthritis with a vibrotactile device applied to the external ear. Bioelectron Med. 2019 Apr 17;5:4.
- Marsal S, Corominas H, De Agustin De Oro JJ, et al. Non-invasive vagus nerve stimulation improves signs and symptoms of rheumatoid arthritis: Results of a pilot study [abstract]. Arthritis Rheumatol. 2020;72 (suppl 10).
- Drewes AM, Brock C, Rasmussen SE, et al. Short-term transcutaneous non-invasive vagus nerve stimulation may reduce disease activity and pro-inflammatory cytokines in rheumatoid arthritis: Results of a pilot study. Scan J Rheumatol. 2021 Jan; 50(1):20–27.
- Courties A, Berenbaum F, Sellam J. Vagus nerve stimulation in musculoskeletal diseases. Joint Bone Spine. 2021 May; 88(3):105149.
- Brock C, Rasmussen SE, Drewes AM, et al. Vagal nerve stimulation-modulation of the anti-inflammatory response and clinical outcome in psoriatic arthritis or anklyosing spondylitis. Mediators Inflamm. 2021 May 27;2021:9933532.
- Courties A, Deprouw C, Maheu E, et al. Effect of transcutaneous vagus nerve stimulation in erosive hand osteoarthritis: Results from a pilot trial. J Clin Med. 2022 Feb 18;11(4):1087.