Kimberly Retzlaff is a freelance medical journalist based in Denver.
References
- Curtis JR, Lee EB, Kaplan IV, et al. Tofacitinib, an oral Janus kinase inhibitor: Analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann Rheum Dis. 2016 May;75(5):831–841.
- Cohen S, Radominski SC, Gomez-Reino JJ, et al. Analysis of infections and all‐cause mortality in phase II, phase III, and long‐term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014 Nov;66(11):2924–2937.
- Winthrop KL, Park S-H, Gul A, et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016 Jun;75(6):1133–1138.
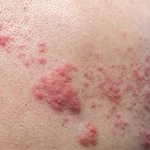
- Winthrop KL, Yamanaka H, Valdez H, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014 Oct;66(10):2675–2684.
- Lilly. Baricitinib (LY3009104): Rheumatoid Arthritis (briefing document). NDA 207924. FDA Advisory Committee Meeting. 2018 Apr 23.
- Brown T. FDA warns of risk for PE, death with higher dose tofacitinib (Xeljanz) for RA. Medscape. 2019 Feb 25.
* Dr. Winthrop has received research funding from Pfizer and BMS; performed consultant work for Amgen, AbbVie, Pfizer, UCB, Genentech, BMS and Lilly; and serves on the data safety monitoring boards for RCTs conducted by UCB, Roche, Astellas, Lilly, Janssen and Galapagos.