“And yet it moves” is a phrase said to have been uttered before the Roman Inquisition by the Italian mathematician, physicist, and philosopher Galileo Galilei in 1633 after being forced to recant his “belief” that the Earth moves around the Sun. As such, the phrase is used today as a sort of pithy retort to imply that it doesn’t matter what you believe, these are the facts.
By analogy to Galileo and his challenge to the “orthodoxy” of accepted measurements, our current trials of biologic therapies in Sjögren’s syndrome (SS) have been based on targeting a variety of cytokines and their receptors. These are targets that have been identified as exhibiting altered expression in blood and glandular tissues. In our clinical trials with biologic agents, we have succeeded in improving a spectrum of extraglandular manifestations and associated biomarkers.
However, the most disabling symptoms of ocular and oral discomfort, fatigue, and myalgias have not shown significant differences in clinical trials with biologic agents.1,2 The fact that we have improved the extraglandular features and biomarkers in these trials should not be minimized. These are the elements that can be easily measured. In our rheumatology clinic, we note that:
- Symptoms of oral or ocular pain do not closely correlate with objective signs of dry eyes or dry mouth.
- Fatigue and myalgia often do not correlate with acute-phase reactants such as erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP).
The situation is far more complicated than treating patients with polymyalgia rheumatica, where we have a wonderful clinical correlation with the ESR and emerge as superheroes in the eyes of patients following their first dose of steroids.
The inadequately explained relationship between objective findings and symptoms in SS is more suggestive of neuropathic pain. Impaired, descending neural inhibition and the resulting failure to control physiologic pain amplification (central sensitization) have been implicated in chronic peripheral pain.
In chronic ocular or oral pain, local inflammation initiates neural stimulation at both the mucosal surface and at the level of the trigeminal nuclei. The integration and cortical processing of those pain signals is not well correlated with markers of systemic inflammation. These neural processes, particularly at the trigeminal nerve and brain cortex, are modulated by chronic exposure to cytokines released from microglial cells.3
By analogy to the quote from Galileo, this is currently the nonmeasurable variable that we must first measure and then use to direct future therapy.
A fascinating article by Rosenthal et al on chronic ocular pain in patients with dry eye following Lasik surgery and in patients with chronic, severe dry-eye syndrome noted several key findings5:
- In patients with acute painful eye (such a corneal abrasion), the ocular pain is largely removed with topical anesthesia.
- In patients with chronic, longstanding ocular pain due to SS, the level of pain (rated on a 1-to-10–point scale) only decreased by a small degree (about 30% to 50%) with topical anesthetic.
- The discrepancy between acute and chronic pain perception is not due to malingering behavior, but to changes in the brain’s mapping of nociceptive signals as a result of chronic stimulation.
In our clinical trials with biologic agents, we have succeeded in improving a spectrum of extraglandular manifestations and associated biomarkers. However, the most disabling symptoms of ocular and oral discomfort, fatigue, and myalgias have not shown significant differences…
Thus, patients with SS are more refractory to symptomatic improvement, and we do not know why. As rheumatologists, we need to formulate better treatment options for our patients with SS. At present, we can summarize some of the major observations and list some of the obstacles toward formulating better treatment algorithms.
- The symptoms most troublesome and disabling to patients with SS—dryness and fatigue—have not responded adequately to a variety of biologic agents.
- Extraglandular manifestations of SS, such as vasculitis and associated biomarkers, have responded to biologic agents, so we know that the biologics are working, at least in part, on the systemic manifestations of SS.
- We can dissociate, at least partly, the pathways of “peripheral” vasculitic inflammation from the pathways that lead to symptoms of oral and/or ocular pain and fatigue.
- Future attention—including animal models—is needed to examine the actions of inflammatory processes on sites of neuropathic pain (trigeminal nerve, dorsal root ganglia, brain) that may not be reflected in the traditional biomarkers or cytokines measured in blood or salivary gland tissue.
Successes in SS: An Oxymoron?
We have learned that cytokines, chemokines, and neurokines are staggeringly complex. In rheumatoid arthritis (RA) and several related arthropathies, initial trials with tumor necrosis factor inhibition were successful beyond our wildest dreams. These successes led to cloning further signaling pathways and additional therapeutic agents for RA.
Patients with multiple sclerosis (MS) and patients with depression frequently complain of dryness as well as fatigue. Instead of simply labeling this malingering behavior, it is an opportunity to understand the models of cortical and subcortical function that we have not addressed in SS. The problems in combining immunologic and neurological approaches are partly bureaucratic, as well as scientific.
Several years ago, I was quite interested in the positive results shown in the fatigue level among patients with systemic lupus erythematosus who were receiving rapamycin and a murine model of SS.1,2 Knowing that there were several issues that needed to be addressed, I did a quick Google search and found that the target for rapamycin is mTor. This molecule is also a critical target for drug development in patients with neuropathy as well as depression.3
Another Google search revealed that an analog of rapamycin had been created by a San Diego–based pharmaceutical company, and that this compound lowered elevated lipids, a very significant issue for patients with lupus and patients with SS. It had been developed and had already undergone phase II trials for potential treatment in MS with good safety results. However, the division that created this analog was subsequently eliminated by this particular pharmaceutical company.
Since a number of physicians who had worked at Scripps Memorial Hospital had migrated to this large pharmaceutical enterprise’s division in San Diego, I eventually found a former member of the now-extinct transplant team who told me that the compound had been licensed to someone else for research related to aging and that nothing more could be done.
Another potential class of compounds with both immunologic and neurologic properties is AKT inhibitors.4,5 I found several compounds that improved fatigue and pain symptoms in development for ulcerative colitis; however, they were being developed by the gastroenterology and neurology divisions of the pharmaceutical company. As such, the compounds were not in the scope of the immunology division unless they proved successful in ulcerative colitis.
Although the company in San Diego was interested in SS research, they wanted an anticytokine biologic molecule. I am not a specialist in drug design or bioinformatics, yet it took only a few moments on Google to locate at least two candidates. Indeed, this experience in trying to identify candidate drugs for SS did stimulate me to write this article to encourage better cooperation between the neurology and immunology divisions at both the clinical and drug-development levels.
Pathogenesis of SS
Primary SS (pSS) is a systemic disorder characterized by dryness that is attributed to lymphocytic infiltrate of lacrimal and salivary glands.7,8 Further, patients with pSS have characteristic antibodies against SS-A and/or SS-B antigens.9,10
There is a misconception that the lymphoid infiltrates lead to progressive destruction and fibrosis of the glandular tissue. However, this view does not really fit the observation that minor salivary-gland biopsies from patients with pSS only have about 50% destruction of the acini and ducts, even in patients with long-standing disease and severe objective dryness. Further, the number of lymphocytic foci does not seem to increase with time, even though many patients note increasing sicca symptoms. The symptoms of dryness and myalgias may increase independent of biomarkers such as ESR, CRP, immunoglobulins, or a variety of cytokines.
We have concentrated therapeutic efforts on pathways involving T-cell and B-cell activation, and these therapies do improve extraglandular manifestations. However, these cytokines also affect intracellular calcium flux in neural receptors and may modulate their associated function. We need to focus our attention on chemokines such as monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), and other agonists of glucagon-related peptide (GCRP) receptors. In addition, neural receptors such as TRPV1, TRPM8, TRPA1, and GCRP are involved in transduction of ocular and oral pain; thus, they should be considered as potential therapeutic targets.6
How We Define Benign, Moderate, and Severe SS
In pSS, clinical features are often divided into two groups:
- Benign subjective symptoms including dryness, arthralgias, myalgias, vague cognitive (executive function) disturbance, and fatigue; and
- Extraglandular or systemic manifestations including rash, synovitis, myositis, vasculitis, pulmonary, renal, neurologic (central and peripheral), hematopoetic (anemia, thrombocytopenia), and lymphoproliferative, including lymphoma.
Although we consider the subjective symptoms to be “benign,” they are the chief cause of patient disability. As noted, the correlation between symptoms and objective signs is extremely poor, and represents one of the unmet needs for future therapies.
Benign Symptoms and the “Functional Circuit”
Although peripheral inflammation may initiate the process, the continued stimulation of the peripheral pain receptors may initiate a series of neurologic processes that become dissociated from the original stimuli. This may occur at either the cortical level or at the level of the spinal cord. We will first review the proposed cortical circuit.
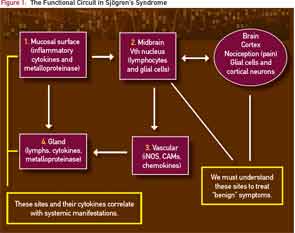
Stern et al introduced the concept of “functional circuit.”11-14 As shown in Figure 1, the mucosal surfaces such as the cornea (or mouth) are blanketed by highly innervated, unmyelinated nerve fibers. These afferent fibers run to the particular areas of the midbrain called the lacrimal and salvatory nuclei.
The nuclei also communicate with particular cortical areas that govern the sensation of dryness and discomfort. Considering the peripheral and cortical inputs, the efferent nerves emerge from these midbrain nuclei and innervate the blood vessels and secretory (lacrimal or salivary) glands.
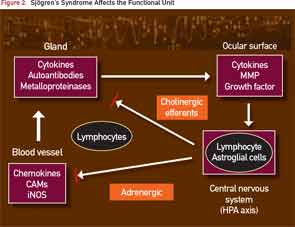
In patients with SS, this is simplified schematically in Figure 2, where the infiltration of lymphocytes into the salivary gland generates an inflammatory milieu with cytokines and metalloproteinases that interfere with glandular transduction of neural signals to activate the secretory process.
Treatment with biologics, including anti-CD20 and anti–B-cell activating factor, decreases the number of lymphocytes in the gland and reduces glandular mRNA transcripts of proinflammatory signals, but there is no corresponding improvement in the patient’s symptoms. The current dilemma is summarized in Figure 2, where the central nervous system is now shown as a connection to the midbrain (cranial nerve V) circuits.
Phantom Pain and Ocular Pain in SS
As noted earlier, ocular symptoms in patients with SS are out of proportion with objective observations of the ocular surface, largely due to the mysterious perception of “phantom pain.”
The concept of “phantom pain” may arise at the level of the cranial nerve, spinal dorsal root ganglia, and thalamus or brain cortex. As noted above, these processes may be strongly influenced by local release of cytokines by microglial cells.3 This observation correlates with our clinical observation that the pain and discomfort in patients with SS whose disease is longstanding is much less improved following treatment with biologic agents than in patients with early disease.
Phantom pain explains the aberrant, painful ocular sensation that chronic post-Lasik patients or patients with SS experience when they report that their pain is only partially relieved by a topical anesthetic.
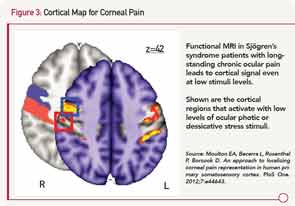
Sites for ocular pain have been mapped by functional magnetic resonance imaging to a specific region of the prefrontal cortex (see Figure 3).15
To emphasize, a patient’s complaints of pain derive not only from the pain fiber on the surface of the eye, but also from the part of the brain where that sensation is processed and remembered. Thus, a new frontier for therapy will be the understanding of noxious stimuli from primary somatosensory cortex that involves alpha-delta fibers.16
I began following the simple procedure of Rosenthal et al in our rheumatology clinic by asking patients with SS about their ocular pain level at their routine exams. Not unexpectedly, patients with longstanding disease would report their pain level to be a seven or 8 on a scale of 10. What was surprising was that patients reported their pain level had diminished only slightly, to a 4 or 5 score after instillation of a topical anesthetic into the eye. There is little doubt that the anesthetic was effective, since the corneal reflex (the blink after gentle pressure to cornea) was obliterated indicating loss of the afferent loop of the reflex.
In summary, it appears that a patient with an acute insult to their eye (such as a corneal abrasion) may have significant, although temporary improvement in ocular discomfort with a topical anesthetic. However, the patient with SS who has chronic eye pain does not obtain the same relief from anesthetic. The simplest explanation is that patients with SS develop a phantom pain as the result of the chronic ocular irritation.
This interaction of different central pathways has been postulated by neuroscientists such as Dr. Ramachandran and colleagues at the University of California at San Diego’s Salk Institute to explain the phantom pain felt in an amputated extremity. His hypothesis is complex, but is nicely summarized at Ted.com/talks and in a YouTube video presentation.
Thus, we can make some immediate suggestions to help get on track to treat the benign symptoms by identifying target molecules:
- Use animal models of SS and their normal congenics (such as nonobese diabetic or thrombospondin, knockout mice) in preclinical studies to screen molecules with antiinflammatory, antidepressant, and antinociceptive agents. I predict that the “immune” animal will have a different dose response due to their inflammatory milieu and danger-signal responses.
- Study the trigeminal nerves in SS models as well as the salivary glands. Examine neurotransmitters at each site to identify new drug-target molecules.
- Investigate the corneal surface and lacrimal function in preclinical models of pain and depression, as these may be abnormal and provide new therapeutic targets.
- Involve neurochemists in the design of clinical therapies for SS.
We began our discussion of SS with the observation that local inflammation in the glands leads to a global dysfunction in secretory ability disproportionate to the number of residual acini and ductal cells. Perhaps this is an important starting point to understand the interface between inflammatory and neural/secretory pathways.
In order to make a significant difference in the quality of life for patients with SS, new therapies must not only improve extraglandular manifestations of SS, but also alleviate the symptoms (fatigue, dryness, and pain) that lead to patient disability.
We must first learn to measure the objective variables that reflect the patient’s symptoms. This will require better cooperation between rheumatologists and experts in the field of neural pain circuits. With a more focused effort, the complex interplay among immune, neural, and hypothalamic pathways in SS may finally be unraveled.
Acknowledgment
The authors gratefully acknowledge helpful discussions with Drs. Perry Rosenthal of Harvard Medical School, Boston, Mass.; Simon Bowman of University Hospitals Birmingham NHS Trust, Birmingham, England; Jules Birnbaum of the Jerome L. Greene Sjögren’s Syndrome Center, Baltimore, Md.; James Cahill of Scripps Memorial Hospital–Ximed, La Jolla, Calif.; and Vilayanur Ramachandran of the University of California at San Diego’s Salk Institute.
Dr. Fox is a rheumatologist and Carla Fox is a registered nurse in the rheumatology clinic at Scripps Memorial Hospital–Ximed in La Jolla, Calif.
References
- Bowman SJ, Booth DA, Platts RG. Measurement of fatigue and discomfort in primary Sjögren’s syndrome using a new questionnaire tool. Rheumatology (Oxford). 2004;43:758-764.
- Bowman SJ, St. Pierre Y, Sutcliffe N, et al. Estimating indirect costs in primary Sjögren’s syndrome. J Rheumatol. 2010; 37:1010-1015.
- Milligan ED, Twining C, Chacur M, et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026-1040.
- Konttinen YT, Hukkanen M, Kemppinen P, et al. Peptide-containing nerves in labial salivary glands in Sjögren’s syndrome. Arthritis Rheum.1992;35:815-820.
- Rosenthal P, Borsook D. The corneal pain system. Part I: The missing piece of the dry eye puzzle. Ocul Surf. 2012;10:2-14.
- Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098-1107.
- Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321-331.
- Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, Bosch X, Tzioufas AG. Topical and systemic medications for the treatment of primary Sjögren’s syndrome. Nat Rev Rheumatol. 2012;8:399-411.
- Theander E, Vasaitis L, Baecklund E, et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren’s syndrome. Ann Rheum Dis. 2011;70:1363-1368.
- Reksten TR, Jonsson MV, Szyszko EA, Brun JG, Jonsson R, Brokstad KA. Cytokine and autoantibody profiling related to histopathological features in primary Sjögren’s syndrome. Rheumatology (Oxford). 2009;48:1102-1106.
- Stern M, Beuerman R, Fox R, Gao J, Mircheff A, Pflugfelder S. The pathology of dry eye: The interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584-589.
- Stern M, Gao J, Siemasko K, Beuerman R, Pflugfelder S. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78:409-416.
- Stern M, Schaumburg C, Dana R, Calonge M, Niederkorn J, Pflugfelder S. Autoimmunity at the ocular surface: Pathogenesis and regulation. Mucosal Immunol. 2010;3:425-442.
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. A unified theory of the role of the ocular surface in dry eye. Adv Exp Med Biol. 1998;438:643-651.
- Moulton EA, Becerra L, Rosenthal P, Borsook D. An approach to localizing corneal pain representation in human primary somatosensory cortex. PloS One. 2012;7:e44643.
- Omori S, Isose S, Otsuru N, et al. Somatotopic representation of pain in the primary somatosensory cortex (S1) in humans. Clin Neurophysiol. 2013;124:1422-1430.
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991-1045.