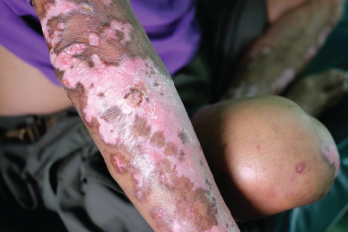
This salt-and-pepper appearance is characteristic of scleroderma.
A yearlong pilot study that evaluated the safety and efficacy of belimumab in a small group of patients with early diffuse systemic sclerosis found no significant difference in the number of adverse events between those treated with the drug and those who received a placebo.
Currently, no drugs are approved specifically for the treatment of systemic sclerosis—also called scleroderma, says Jessica Gordon, MD, MSc, assistant attending physician for the Department of Rheumatology at the Hospital for Special Surgery (HSS) in New York City and first author of the study published recently in Arthritis & Rheumatology. A human monoclonal antibody that inhibits B cell activating factor, belimumab (Benlysta)—an FDA-approved treatment for lupus—was chosen as a candidate for the pilot study because of the important role B cells play in the pathogenesis of systemic sclerosis.
“There are similarities between scleroderma and lupus, including a significant role for B cells, so in this trial we investigated whether we could reappropriate a treatment that was already approved for lupus,” explains Dr. Gordon.
Background
Scleroderma is a rare autoimmune connective tissue disease that causes thickening of the skin and leads to complications for the lungs, heart, kidney and other organs, as well as vascular dysfunction, as often evidenced early on by Raynaud’s phenomenon, which causes numbing and coldness in the hands and feet. Patients may experience trouble breathing, cardiac arrhythmia, gastrointestinal difficulties and joint pain.

Dr. Gordon
The disease is also highly heterogeneous: Organ system involvement and disease manifestation tend to differ from one patient to the next, says Dr. Gordon.
“One patient may have very severe skin or musculoskeletal disease,” she says. “Another patient may have very mild skin disease but could have severe lung disease.”
The Study
The industry-sponsored study involved 20 patients in a randomized, double-blind, placebo-controlled trial conducted by an investigator at a single center at HSS. All participants were 18 years or older, met ACR preliminary criteria for systemic sclerosis and had early diffuse systemic sclerosis of three years’ or shorter duration.
The primary objective was to assess the safety and tolerability of belimumab in patients by comparing the number of adverse events between the belimumab and placebo groups. The primary efficacy endpoint was the difference in skin thickness as assessed by the modified Rodnan skin score (MRSS) after 52 weeks.
Secondary efficacy endpoints were evaluated with the use of surveys and questionnaires. Skin biopsies and serum B-lymphocyte stimulator levels also were assessed.
Early disease patients were recruited because skin problems may regress in later stages, making it more difficult to assess whether therapy is working, says Dr. Gordon. This approach also addressed a desire to explore possibilities for prevention of disease progression, she says.
In addition to treatment with belimumab, the study design called for patients to receive a background therapy of mycophenolate mofetil, a drug commonly used to treat autoimmune diseases, says Dr. Gordon. In recent years, evidence for the use of mycophenolate in scleroderma has been increasing, suggesting it improves skin disease and stabilizes lung function—although its use is still considered off label, she says.
“We didn’t want to ask [patients] to just take placebo without any background therapy,” because study participants had active disease, explains Dr. Gordon.
After a wash-in period, patients were randomized to receive intravenous doses of 10 mg/kg of belimumab or placebo at two-week intervals for the first three doses and then at four-week intervals up to Week 48. They also continued with twice daily treatments of mycophenolate mofetil at 1,000 mg per dose and returned for final assessment at Week 52.
Most patients were female, which is representative of the condition, and on average had been symptomatic for less than one year. In this study, patients had severe widespread skin involvement, covering the trunk, thighs and upper arms, and a small percentage had significant interstitial lung disease, says Dr. Gordon.
“A significant portion of the patients were positive for the anti-RNA polymerase 3 antibody, which is an autoantibody in systemic sclerosis associated with rapidly progressive and severe skin disease,” notes Dr. Gordon.
Results
Researchers found no significant difference in the number of adverse events between the groups, with 53 in the belimumab group and 56 in the placebo group.
Dr. Gordon says this was the first time these patients had been exposed to belimumab, and the results indicate the safety profile was similar to what is seen in lupus treatment, meaning there were no unexpected adverse events.
“The safety profile was reassuring,” says Dr. Gordon, but she notes that “additional studies are needed to determine if belimumab has a role in the treatment of diffuse scleroderma.”
Among patients in the belimumab group, there was a trend toward greater improvement in skin thickness based on the MRSS, but there was no significant difference in the median score compared with the placebo group, meaning the primary efficacy endpoint was not met. Additionally, patients with improved MRSS in the belimumab group had a significant decline in expression of B cell signaling and profibrotic genes and pathways not seen in the placebo group.
“I see this sort of work as seeking to understand biology and generating new hypotheses that we can go forward with,” says Dr. Gordon.
Limitations & Future Outlook
The study’s small sample size was one of its limitations, and it was underpowered to detect modest differences between cohorts, the authors write. Use of the comparator minimized ability to detect whether belimumab affected clinical outcomes.
In summarizing the study, Dr. Gordon says that, overall, the safety findings were as expected; the skin score results were interesting, even though statistical significance was not achieved; and secondary measures were negative for the most part. This line of study is important to pursue because systemic sclerosis has both the highest mortality and the highest rate of disability of all the rheumatic diseases, she says.
In the past 10 to 20 years, Dr. Gordon says, rheumatologists have “seen dramatic improvements in the lives of many of our patients. Some examples are those with rheumatoid arthritis or psoriatic arthritis who have multiple treatment options. Patients’ experience of those illnesses today vs. 20 years ago is dramatically different.
“Although we’ve made really important strides in the understanding of systemic sclerosis, this condition continues to be a particularly difficult one to treat,” she continues. “The condition is rare and heterogeneous, so it can be challenging to study. Patients with systemic sclerosis urgently need improved therapies. That is a major motivation to look at novel medicines.”
Catherine Kolonko is a medical writer based in Oregon.
Reference
- Gordon JK, Martyanov V, Franks JM, et al. Belimumab for the treatment of early diffuse systemic sclerosis. Results of a randomized, double-blind, placebo-controlled, pilot trial. Arthritis Rheumatol. 2018 Feb;70(2):308–316.


