WASHINGTON, D.C.—Retroperitoneal fibrosis (RPF) is often a challenging diagnosis to make, given a lack of serologic biomarkers and often difficulty in accessing tissue for biopsy, especially in cases confined to the retroperitoneum. The topic of retroperitoneal fibrosis was discussed during the CARE to Test Your Knowledge: Retroperitoneal Fibrosis session at ACR Convergence 2024.
John Stone, MD, MPH, professor of medicine at Harvard Medical School, Boston, the Edward A. Fox Chair in Medicine at Massachusetts General Hospital (MGH), Boston, and the executive chair of the IgG4ward! Foundation, provided an overview of an approach to RPF and important considerations in its diagnosis and management.
4 Management Principles
Dr. Stone noted four principles that rheumatologists should know about RPF. The first principle is that most cases of idiopathic RPF are probably IgG4-related disease (IgG4-RD), or at least should be treated that way. The second principle is that there is an important differential diagnosis that one must cautiously work through.
“The differential diagnosis includes, most importantly, malignancy, such as sarcoma, lymphoma or metastatic carcinoma, which can mimic retroperitoneal fibrosis,” Dr. Stone said. Primary amyloidosis, Erdheim-Chester disease, sarcoidosis or tuberculosis are also entities in the differential that may mimic RPF (see Table 1).
His third principle is that a lot remains to be known about RPF.
And finally, the fourth principle is to not fear RPF: “This disease … is responsive to therapy and patients do well,” Dr. Stone said.
| Table 1: RPF DDx: Mimics of Retroperitoneal Fibrosis |
|---|
| Malignancy • sarcoma • lymphoma • metastatic carcinoma |
| Primary Amyloidosis |
| Erdheim-Chester Disease |
| Sarcoidosis |
| Tuberculosis |
RPF on a Spectrum with IgG4-RD
Dr. Stone then made a case for why RPF should be considered as part of the spectrum of IgG4-RD, which he noted may seem surprising as RPF often feels different than IgG4-RD. Unlike IgG4-RD, patients with RPF have typically normal serum IgG4 levels, are serologically inactive (with absence of hypocomplementemia, normal IgE levels), often have single-organ involvement (confined to the retroperitoneum) and are slowly responsive to therapy.
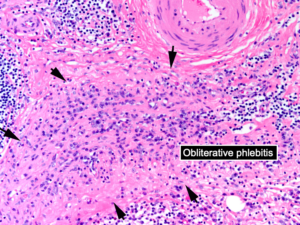
Despite these clinical differences, Dr. Stone argued that pathology is a clue linking these two entities, given similar histopathologic findings on biopsy. IgG4-RD is characterized by lymphoplasmacytic infiltrate on biopsy with many plasma cells staining for IgG4 and often a distinct pattern of storiform fibrosis and obliterative phlebitis of blood vessels, particularly veins (see Figure 1). “This is exactly what is seen in retroperitoneal fibrosis as well,” Dr. Stone said.