Pipeline and Drug Approvals
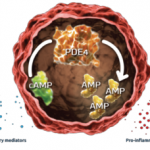
A new drug application (NDA) has been submitted to the Food and Drug Administration (FDA) for apremilast (CC-10004) to treat psoriatic arthritis.1 An FDA response is expected sometime this month. Apremilast is an oral phosphodiesterase 4 (PDE4) inhibitor that regulates inflammatory and immune processes.2 It has been shown to quell a number of different cytokines (e.g., tumor necrosis factor–alfa, interleukin [IL-] 12, IL-23, and interferon-gamma) and chemokines, improving a patient’s inflammatory response. The manufacturer of apremilast has also submitted an NDA for the drug to treat psoriasis.3 Rheumatoid arthritis (RA) and ankylosing spondylitis (AS) trials are currently in phase III. Results of the psoriatic arthritis trials were recently presented at the ACR/ARHP Annual Meeting in San Diego. The first phase III trials (PALACE 1, 2, and 3) compared apremilast to placebo in patients who had previously been treated with biologic and nonbiologic disease-modifying antirheumatic drugs (DMARDs). In the phase III “Efficacy and Safety Study of Apremilast to Treat Active Psoriatic Arthritis (PALACE 2)” trial, patients were allowed to continue DMARDs, including sulfasalazine, leflunomide, methotrexate (MTX), or a combination of these.4 There were 159 patients randomized to received placebo, 163 patients in the 20-mg twice-daily (BID) apremilast treatment arm, and 162 patients in the 30-mg BID apremilast treatment arm. After Week 16, placebo-treated patients were randomized to an active treatment group. Following one year of treatment, the Psoriasis Area and Severity Index (PASI) 50/PASI 75 was achieved by 49% of the 20-mg BID–treated patients and 59% of the 30-mg BID–treated patients. Adverse reactions were mild to moderate, and most patients did not stop treatment due to these adverse reactions. Adverse reactions occurred in 5% of patients and included nausea, diarrhea, and headache. PALACE 4 utilized apremilast 20 mg BID for a 16-week treatment duration. Twenty-nine percent of patients achieved an ACR 20 response.1 In patients who received 30 mg BID, 32% achieved an ACR 20 response, and placebo-treated patients had a 17% response. After Week 52 of treatment, 53% of the 20-mg BID–treated group achieved an ACR 20, and 59% of the 30-mg BID–treated group achieved an ACR 20. In addition, 14% and 18%, respectively, achieved an ACR 70 response at Week 52.
Diclofenac sodium topical solution (Pennsaid 2%) has been FDA approved as a BID nonsteroidal antiinflammatory drug (NSAID) to treat pain associated with osteoarthritis (OA) of the knee.5 This formulation carries the same risks as other oral and topical NSAIDs, including cardiovascular and gastrointestinal risks and liver function test (LFT) elevations. This is a follow-on product to the diclofenac sodium 1.5% topical solution product. It has a higher strength than the former product and is more viscous.