ajt/shutterstock.com
On Nov. 19, 2015, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended granting marketing authorization for SB4, an etanercept biosimilar product that will be called Benepali.1 On Jan. 16, 2016, EMA granted marketing authorization in the European Union for Benepali to be used to treat rheumatoid arthritis (RA), psoriatic arthritis (PsA), non-radiographic axial spondyloarthritis and plaque psoriasis.2 Benapali is an etanercept biosimilar and the first subcutaneous anti-TNF biosimilar available in the European market.
On Nov. 25, 2015, Amgen submitted an application for marketing approval to the U.S. Food and Drug Administration (FDA) for ABP 501, its adalimumab biosimilar.3 The submission includes analytical, clinical and pharmacokinetic data. Phase 3 safety and comparative efficacy studies were performed for both moderate to severe plaque psoriasis and moderate to severe RA. The study results showed clinical equivalence to adalimumab, with safety and immunogenicity comparable with adalimumab. On Jan. 5, 2016, the FDA accepted this application for review.
Worldwide, Humira (adalimumab) sales were almost $12.5 billion in 2014. Humira patents expire in December 2016 in the U.S. and April 2018 in Europe.
Apremilast Proves Effective for PsA with Skin Involvement
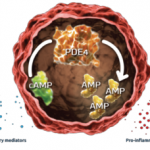
Apremilast, a phosphodiesterase 4 inhibitor, is already approved to treat patients with psoriatic arthritis (PsA) and continues to be studied in different populations and at different doses.4 A recent study by Christopher J. Edwards, BSc, MBBS, MD, FRCP, associate director of the Southampton NIHR Wellcome Trust Clinical Research Facility, University Hospital Southampton in the UK, and colleagues evaluated the safety and efficacy of apremilast in treating patients with active psoriatic arthritis (PsA) and current skin involvement, despite treatment with conventional disease-modifying anti-rheumatic drugs (DMARDs) and/or biologic DMARDs.5
This study, known as PALACE 3 (Psoriatic Arthritis Long-term Assessment of Clinical Efficacy), screened 612 patients and enrolled in the study 505 adults with PsA for six or more months who also met the Classification Criteria for Psoriatic Arthritis (CASPAR) at screening and had at least three swollen and tender joints. Patients were randomized to receive 20 mg apremilast twice daily, 30 mg apremilast twice daily or placebo. If placebo-treated patients did not achieve a 20% improvement in swollen and tender joint counts by Week 16, they received apremilast rescue treatment. At Week 24, the remaining placebo patients were then randomized to receive either 20 mg or 30 mg apremilast twice daily.
The FDA has approved a supplemental New Drug Application for tofacitinib (Xeljanz 5 mg), eliminating the Risk Evaluation & Mitigation Strategy (REMS) requirement that was initially approved on Nov. 6, 2012, & modified on June 19, 2015.
Safety and efficacy were assessed throughout the 52-week study. The ACR20 response was the primary efficacy endpoint. At Week 16, it was achieved in significantly more patients who received 30 mg apremilast twice daily and those who received 20 mg apremilast twice daily compared with placebo-treated patients (41%, 28% and 18%, respectively; P<0.0001). The mean decrease in the Health Assessment Questionnaire Disability Index (HAQ-DI) score was a key secondary endpoint. The HAQ-DI was achieved in a significantly greater number of patients treated with 30 mg apremilast twice daily compared with placebo-treated patients. At Week 16, 41% of patients treated with 30 mg apremilast achieved PASI-50 compared with 24% of placebo-treated patients. This result was statistically significant. For the 20-mg-apremilast-treated patients at Week 16, 33% achieved PASI-50, which was numerically higher, but not statistically significant.