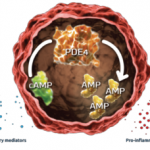
Additionally, at Week 16, significantly more patients treated with 20 mg or 30 mg apremilast achieved PASI-75 compared with placebo-treated patients (20%, 21% and 8%, respectively).
In patients who continued therapy, sustained efficacy was demonstrated at Week 52. Most adverse events were mild to moderate in severity, the most common of which were diarrhea, headache, nausea and upper respiratory tract infection.
Tofacitinib Released from FDA REMS Requirement
The U.S. Food and Drug Administration (FDA) has approved a supplemental New Drug Application (sNDA) for tofacitinib (Xeljanz 5 mg), eliminating the Risk Evaluation and Mitigation Strategy (REMS) requirement that was initially approved on Nov. 6, 2012, and modified on June 19, 2015.6 The REMS consisted of a communication plan and a timetable for submission of REMS assessments.
CHS-0214 Shows Biosimilarity to Etanercept CHS-0214, a proposed etanercept (Enbrel) biosimilar, met its primary endpoint of ACR20 at Week 24 in a Phase 3 clinical trial.7 In this study, CHS-0214 was compared with the safety and efficacy of etanercept in patients with moderate to severe rheumatoid arthritis (RA) that was inadequately controlled with methotrexate (MTX) monotherapy.
Compared with etanercept, CHS-0214 had no clinically meaningful differences in safety and immunogenicity. This current study will continue for 52 weeks. It’s the second Phase 3 trial of CHS-0214, which is manufactured by Baxalta.
In November 2015, CHS-0214 met its primary endpoints for the treatment of patients with chronic plaque psoriasis.
Michele B. Kaufman, PharmD, CGP, RPh, is a freelance medical writer based in New York City and a pharmacist at New York Presbyterian Lower Manhattan Hospital.
References
- Pro Pharma Communications International. EMA recommends approval of etanercept biosimilar. GaBI Online. 2015 Nov 27.
- Benepali, the first etanercept biosimilar referencing Enbrel, approved in the European Union. 2016 Jan 16.
- Pro Pharma Communications International. Amgen submits biosimilar adalimumab application to FDA. GaBI Online. 2015 Nov 27.
- National Psoriasis Foundation. About psoriasis: New oral treatments.
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: A phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016 Jan 20. pii: annrheumdis-2015-207963. [Epub ahead of print]
- Han DH. Xeljanz released from REMS requirement. MPR. 2016 Feb 10.
- Anekwe L. Baxalta’s Enbrel biosimilar measures up in late-stage study. Pharmafile.com. 2016 Jan 13.