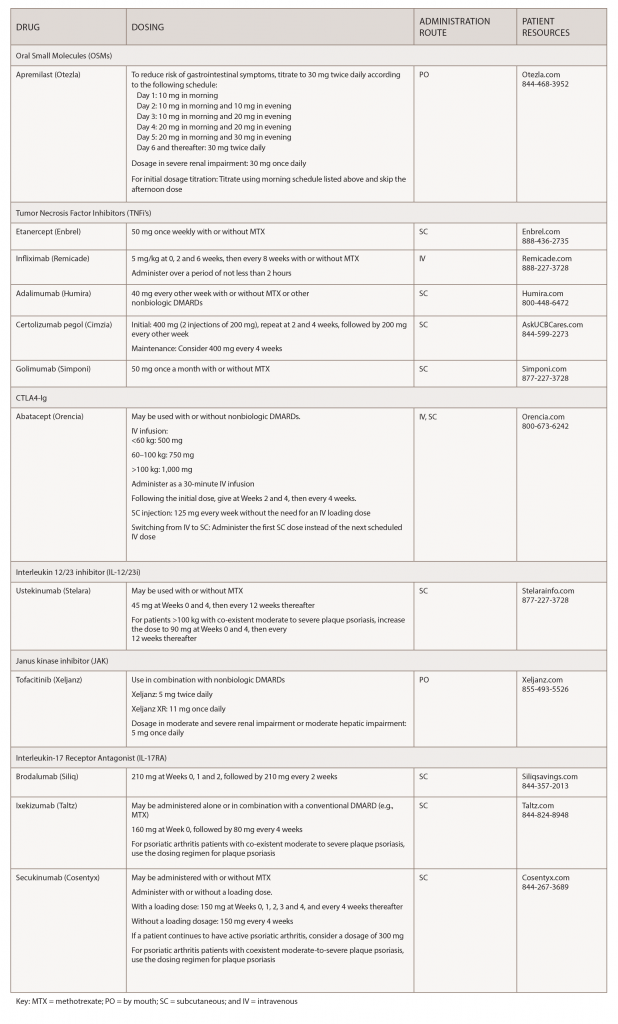
Over the past few years, biosimilars and other new drugs have been introduced to treat rheumatic illnesses. Some of the conditions we treat have numerous drug options, others have few or only off-label options. This series, Rheumatology Drugs at a Glance, provides streamlined information on the administration of biologic, biosimilar and other medications used to treat patients with rheumatic disease. In Part 1 of this series, we discuss the 19 available options to treat psoriatic arthritis (see Table 1).
Psoriatic arthritis (PsA) is a chronic inflammatory musculoskeletal disease that remains undertreated in patients. Clinicians and patients can now choose from a wide variety of pharmacological therapies to control the signs and symptoms, and improve physical function and quality of life by slowing the disease progression and saving joints and other tissues from permanent damage. Standard and biologic disease-modifying anti-rheumatic drugs (DMARDs) may reduce symptoms and attempt to slow PsA disease progression, while non-steroidal anti-inflammatory drugs (NSAIDs) and steroids can be used for pain management and to reduce joint swelling.1
Early diagnosis of PsA and prompt initiation of therapy are important to improve long-term outcomes. Even a six-month delay from symptom onset to the first visit with a rheumatologist contributes to the development of peripheral joint erosions and can worsen long-term physical function.2
Adherence to treatment is crucial for patients to achieve and maintain remission. But patient dissatisfaction with treatment options is apparent when they don’t see a physician, initiate or continue therapies. Concerns with long-term safety, adverse events, administration challenges and cost abound.3 Additionally, patients may feel better after treatment and decide to discontinue their medications. However, patients who stop their medications report their disease returns within three to six months. Unfortunately, a patient’s chance of achieving drug-free remission is low.4
It is important to advise patients that remission does not equal cure. Once patients stop their medications, the symptoms that return may be worse than before.
An integral part of patient care includes the healthcare provider incorporating patient education into their care and understanding PsA from the patient’s perspective. Patient education includes the provision of educational activities related to the disease, treatment and the consequences of non-adherence.5 A discussion of long-term outcomes is often a highly effective way to encourage adherence. A discussion of potential side effects can help prevent a patient from discontinuing treatment prematurely. Advise patients of the need to stay on treatment to avoid recurrences or flares. It is also important physicians review the signs and symptoms of infections with patients and advise patients to let the provider know if they are experiencing any new infections. Most of the biologic treatments require testing for some infections or pre-vaccination to prevent infections once on treatment.
Treatments for PsA can be expensive, and healthcare providers can help make treatment costs more manageable for patients. A number of financial assistance resources are available to patients, and patients can contact the manufacturer of a medication to request co-pay assistance. Your staff can also explore patient assistance programs for those who do not have insurance.6
Depending on a patient’s healthcare coverage and the medication(s) they use, a specialty pharmacy may dispense the medications. Most specialty pharmacies have clinical pharmacists available who can help patients deal with insurance issues, drug monitoring for adverse effects and effectiveness, and answer medication- or disease-related questions.
For additional information, the ACR and the National Psoriasis Foundation (NPF) have published their first joint guideline for treating psoriatic arthritis. It can be found here.1
Apremilast (Otezla):7 Tablets
Drug class: DMARD, phosphodieasterase 4 (PDE4) inhibitor
Warnings & Precautions
- Depression: Individuals should be alerted to watch for the emergence or worsening of depression, suicidal thoughts or other mood changes. If these changes occur, they should contact their physician. Carefully weigh the risks and benefits of apremilast treatment in patients with a history of depression and/or suicidal thoughts or behavior.
- Weight decrease: Weight should be regularly monitored. If unexplained or clinically significant weight loss occurs, consider discontinuing apremilast.
- Drug interactions: Using apremilast with strong cytochrome P450 (CYP 450) enzyme inducers (e.g., rifampin, phenobarbital, carbamazepine, phenytoin) is not recommended due to potential loss of efficacy.
Commentary: The safety and effectiveness of apremilast was evaluated in three clinical trials involving 1,493 patients with active PsA. In the trials, study participants were randomly assigned placebo or 20 or 30 mg apremilast twice daily. Patients were able to continue with DMARDs, low-dose corticosteroids or NSAIDs during the trial. The primary endpoint was ACR 20 response at Week 16. Apremilast plus DMARDs compared with placebo plus DMARDs was associated with greater improvement in signs and symptoms of PsA. Evidence of greater improvement in physical function was also apparent with apremilast (30 mg twice daily) compared with placebo. The most common adverse reactions (≥5%) are diarrhea, nausea and headache.
*Important Safety Information (ISI)
This ISI is applicable to all tumor necrosis factor inhibitors (TNFi’s) and some other immune modulators.
Serious Infections & Malignancies
- There is an increased risk of serious infections leading to hospitalization or death, including tuberculosis (TB), bacterial sepsis, invasive fungal infections (e.g., histoplasmosis) and infections due to other opportunistic pathogens. If these develop, discontinue the drug.
- Before starting treatment, perform a test for latent tuberculosis (TB); if positive, start treatment for TB prior to starting the drug. Monitor all patients for development of active TB during treatment, even if the initial latent TB test was negative.
- Lymphoma and other malignancies, some fatal, have been reported in children and adolescents treated with TNFi’s.
Etanercept (Enbrel):8 Injection
Biosimilar(s): etanercept-szzs (Erelzi)9
Drug class: DMARD, TNFi
Boxed warning: *Refer to *ISI (above)
Warnings & Precautions
- Do not start etanercept during an active infection. If an infection develops, monitor carefully, and stop etanercept if the infection becomes serious.
- Consider empiric anti-fungal therapy for patients at risk (those who reside in or travel to regions where mycoses are endemic) for invasive fungal infections or who develop a severe systemic illness on etanercept.
- Demyelinating disease, exacerbation or new onset, may occur.
- Cases of lymphoma have been observed in patients receiving TNFi’s.
- Congestive heart failure, worsening or new onset, may occur.
- Advise patients to seek immediate medical attention if symptoms of pancytopenia or aplastic anemia develop, and consider stopping etanercept.
- Monitor hepatitis B virus carriers for reactivation during and following therapy. If reactivation occurs, consider stopping etanercept and beginning anti-viral therapy.
- Anaphylaxis or serious allergic reactions may occur.
- Stop etanercept if lupus-like syndrome or autoimmune hepatitis develops.
Commentary: The U.S. Food and Drug Administration (FDA) approved etanercept for the reduction of signs and symptoms in patients with active PsA. The approval was based on two clinical trials, both comparing the efficacy of etanercept vs. placebo. The most common adverse reactions (≥5%) are infections and injection-site reactions.
Infliximab (Remicade):10 Infusion
Biosimilar(s): infliximab-dyyb (Inflectra),11 infliximab-abda (Renflexis),12