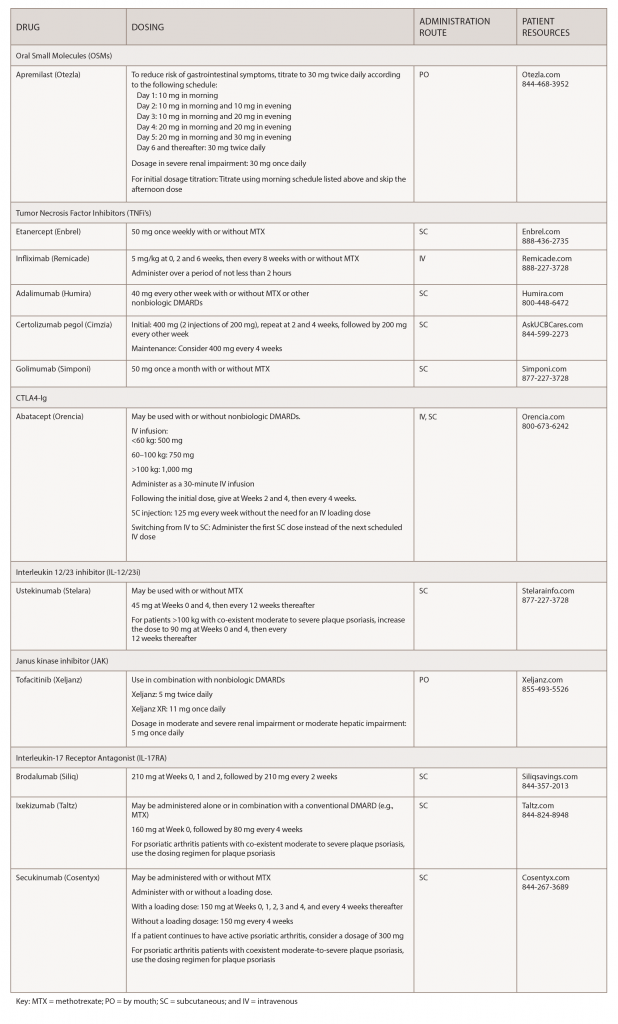
Over the past few years, biosimilars and other new drugs have been introduced to treat rheumatic illnesses. Some of the conditions we treat have numerous drug options, others have few or only off-label options. This series, Rheumatology Drugs at a Glance, provides streamlined information on the administration of biologic, biosimilar and other medications used to treat patients with rheumatic disease. In Part 1 of this series, we discuss the 19 available options to treat psoriatic arthritis (see Table 1).
Psoriatic arthritis (PsA) is a chronic inflammatory musculoskeletal disease that remains undertreated in patients. Clinicians and patients can now choose from a wide variety of pharmacological therapies to control the signs and symptoms, and improve physical function and quality of life by slowing the disease progression and saving joints and other tissues from permanent damage. Standard and biologic disease-modifying anti-rheumatic drugs (DMARDs) may reduce symptoms and attempt to slow PsA disease progression, while non-steroidal anti-inflammatory drugs (NSAIDs) and steroids can be used for pain management and to reduce joint swelling.1
Early diagnosis of PsA and prompt initiation of therapy are important to improve long-term outcomes. Even a six-month delay from symptom onset to the first visit with a rheumatologist contributes to the development of peripheral joint erosions and can worsen long-term physical function.2
Adherence to treatment is crucial for patients to achieve and maintain remission. But patient dissatisfaction with treatment options is apparent when they don’t see a physician, initiate or continue therapies. Concerns with long-term safety, adverse events, administration challenges and cost abound.3 Additionally, patients may feel better after treatment and decide to discontinue their medications. However, patients who stop their medications report their disease returns within three to six months. Unfortunately, a patient’s chance of achieving drug-free remission is low.4
It is important to advise patients that remission does not equal cure. Once patients stop their medications, the symptoms that return may be worse than before.
An integral part of patient care includes the healthcare provider incorporating patient education into their care and understanding PsA from the patient’s perspective. Patient education includes the provision of educational activities related to the disease, treatment and the consequences of non-adherence.5 A discussion of long-term outcomes is often a highly effective way to encourage adherence. A discussion of potential side effects can help prevent a patient from discontinuing treatment prematurely. Advise patients of the need to stay on treatment to avoid recurrences or flares. It is also important physicians review the signs and symptoms of infections with patients and advise patients to let the provider know if they are experiencing any new infections. Most of the biologic treatments require testing for some infections or pre-vaccination to prevent infections once on treatment.
Treatments for PsA can be expensive, and healthcare providers can help make treatment costs more manageable for patients. A number of financial assistance resources are available to patients, and patients can contact the manufacturer of a medication to request co-pay assistance. Your staff can also explore patient assistance programs for those who do not have insurance.6
Depending on a patient’s healthcare coverage and the medication(s) they use, a specialty pharmacy may dispense the medications. Most specialty pharmacies have clinical pharmacists available who can help patients deal with insurance issues, drug monitoring for adverse effects and effectiveness, and answer medication- or disease-related questions.
For additional information, the ACR and the National Psoriasis Foundation (NPF) have published their first joint guideline for treating psoriatic arthritis. It can be found here.1
Apremilast (Otezla):7 Tablets
Drug class: DMARD, phosphodieasterase 4 (PDE4) inhibitor
Warnings & Precautions
- Depression: Individuals should be alerted to watch for the emergence or worsening of depression, suicidal thoughts or other mood changes. If these changes occur, they should contact their physician. Carefully weigh the risks and benefits of apremilast treatment in patients with a history of depression and/or suicidal thoughts or behavior.
- Weight decrease: Weight should be regularly monitored. If unexplained or clinically significant weight loss occurs, consider discontinuing apremilast.
- Drug interactions: Using apremilast with strong cytochrome P450 (CYP 450) enzyme inducers (e.g., rifampin, phenobarbital, carbamazepine, phenytoin) is not recommended due to potential loss of efficacy.
Commentary: The safety and effectiveness of apremilast was evaluated in three clinical trials involving 1,493 patients with active PsA. In the trials, study participants were randomly assigned placebo or 20 or 30 mg apremilast twice daily. Patients were able to continue with DMARDs, low-dose corticosteroids or NSAIDs during the trial. The primary endpoint was ACR 20 response at Week 16. Apremilast plus DMARDs compared with placebo plus DMARDs was associated with greater improvement in signs and symptoms of PsA. Evidence of greater improvement in physical function was also apparent with apremilast (30 mg twice daily) compared with placebo. The most common adverse reactions (≥5%) are diarrhea, nausea and headache.
*Important Safety Information (ISI)
This ISI is applicable to all tumor necrosis factor inhibitors (TNFi’s) and some other immune modulators.
Serious Infections & Malignancies
- There is an increased risk of serious infections leading to hospitalization or death, including tuberculosis (TB), bacterial sepsis, invasive fungal infections (e.g., histoplasmosis) and infections due to other opportunistic pathogens. If these develop, discontinue the drug.
- Before starting treatment, perform a test for latent tuberculosis (TB); if positive, start treatment for TB prior to starting the drug. Monitor all patients for development of active TB during treatment, even if the initial latent TB test was negative.
- Lymphoma and other malignancies, some fatal, have been reported in children and adolescents treated with TNFi’s.
Etanercept (Enbrel):8 Injection
Biosimilar(s): etanercept-szzs (Erelzi)9
Drug class: DMARD, TNFi
Boxed warning: *Refer to *ISI (above)
Warnings & Precautions
- Do not start etanercept during an active infection. If an infection develops, monitor carefully, and stop etanercept if the infection becomes serious.
- Consider empiric anti-fungal therapy for patients at risk (those who reside in or travel to regions where mycoses are endemic) for invasive fungal infections or who develop a severe systemic illness on etanercept.
- Demyelinating disease, exacerbation or new onset, may occur.
- Cases of lymphoma have been observed in patients receiving TNFi’s.
- Congestive heart failure, worsening or new onset, may occur.
- Advise patients to seek immediate medical attention if symptoms of pancytopenia or aplastic anemia develop, and consider stopping etanercept.
- Monitor hepatitis B virus carriers for reactivation during and following therapy. If reactivation occurs, consider stopping etanercept and beginning anti-viral therapy.
- Anaphylaxis or serious allergic reactions may occur.
- Stop etanercept if lupus-like syndrome or autoimmune hepatitis develops.
Commentary: The U.S. Food and Drug Administration (FDA) approved etanercept for the reduction of signs and symptoms in patients with active PsA. The approval was based on two clinical trials, both comparing the efficacy of etanercept vs. placebo. The most common adverse reactions (≥5%) are infections and injection-site reactions.
Infliximab (Remicade):10 Infusion
Biosimilar(s): infliximab-dyyb (Inflectra),11 infliximab-abda (Renflexis),12
infliximab-qbtx (Ixifi)13
Drug class: DMARD, TNFi
Boxed warning: Refer to *ISI (left) and
- Fatal hepatosplenic T-cell lymphoma (HSTCL) has been reported in patients treated with TNFi’s, post-marketing. All infliximab cases occurred in inflammatory bowel disease (IBD) patients, who were mostly adolescent or young adult males. All had received azathioprine or 6-mercaptopurine concomitantly with infliximab at or prior to diagnosis.
Warnings & Precautions
- Do not give infliximab during an active infection. If an infection develops, monitor carefully and stop infliximab if infection becomes serious.
- Invasive fungal infections have occurred. For patients who develop a systemic illness on infliximab, consider empiric antifungal therapy for those who reside in or travel to regions where mycoses are endemic.
- The incidence of malignancies including lymphoma was greater in infliximab-
treated patients than in controls. Due to the risk of HSTCL, carefully assess the risk/benefit especially in Crohn’s disease (CD) or ulcerative colitis patients, in males, and if receiving azathioprine or 6-mercaptopurine treatment.
- Hepatitis B virus (HBV) reactivation can occur. Therefore, test for HBV infection before starting infliximab. Monitor HBV carriers during and several months after therapy. If reactivation occurs, stop infliximab and begin anti-viral therapy.
- Hepatotoxicity—rare severe hepatic reactions, some fatal or necessitating liver transplantation—have occurred. Stop infliximab in cases of jaundice and/or marked liver enzyme elevations.
- Heart failure—new onset or worsening symptoms may occur.
- Cytopenias have occurred. Advise patients to seek immediate medical attention if signs and symptoms develop, and consider stopping infliximab.
- Hypersensitivity—serious infusion reactions, including anaphylaxis, or serum sickness-like reactions may occur.
- Demyelinating disease—exacerbation or new onset may occur.
- Lupus-like syndrome—stop infliximab if the syndrome develops.
- Live vaccines or therapeutic infectious agents should not be given with infliximab.
Commentary: The FDA approved infliximab for treatment of active PsA based on data from two clinical trials that showed positive results. The most common adverse reactions (≥10%) are infections (e.g., upper respiratory, sinusitis, pharyngitis), infusion-related reactions, headache and abdominal pain.
Adalimumab (Humira):14 Injection
Biosimilars: adalimumab-atto (Amjevita),15 adalimumab-adbm (Cyltezo),16 adalimumab-adaz (Hyrimoz)17
Drug class: DMARD, TNFi
Boxed warning: Refer to *ISI (left) and
- Fatal hepatosplenic T-cell lymphoma (HSTCL) have occurred postmarketing in adolescent and young adults IBD patients treated with TNFi’s.
Warnings & Precautions
- Do not start adalimumab during an active infection. If an infection develops, monitor carefully, and stop adalimumab if the infection becomes serious.
- Invasive fungal infections—For patients who develop a systemic illness on adalimumab, consider empiric antifungal therapy for those who reside in or travel to regions where mycoses are endemic.
- The incidence of malignancies was greater in adalimumab-treated patients than in controls.
- Anaphylaxis or serious allergic reactions may occur.
- HBV reactivation can occur. Monitor HBV carriers during and for several months after therapy. If reactivation occurs, stop adalimumab and begin anti-viral therapy.
- Demyelinating disease, exacerbation or new onset, may occur.
- Cytopenias, pancytopenia—advise patients to seek immediate medical attention if symptoms develop, and consider stopping adalimumab.
- Heart failure, worsening or new onset, may occur.
- Lupus-like syndrome—stop adalimumab if the syndrome develops.
Commentary: Adalimumab received an expanded indication based on results from a clinical trial of 313 patients with moderate to severe PsA who had an inadequate response to NSAIDs. Patients taking adalimumab experienced significantly less joint damage than patients taking placebo, and nearly 60% of patients achieved an ACR 20 response through Week 24.The most common adverse reactions (≥10%) are infections (e.g., upper respiratory, sinusitis), injection-site reactions, headache and rash.
Certolizumab pegol (Cimzia):18 Injection
Drug class: TNF inhibitor
Boxed warning: Refer to *ISI (p. 17)
Warnings & Precautions
- Do not start certolizumab during an active infection. If an infection develops, monitor carefully, and stop certolizumab if the infection becomes serious.
- Invasive fungal infections may occur. For patients who develop a systemic illness on certolizumab, consider empiric antifungal therapy for those who reside in or travel to regions where mycoses are endemic.
- Cases of lymphoma and other malignancies have been observed in patients receiving TNFi’s.
- Heart failure, worsening or new onset, may occur.
- Anaphylaxis or serious allergic reactions may occur.
- HBV reactivation can occur. Test for HBV infection before starting certolizumab. Monitor HBV carriers during and several months after therapy. If reactivation occurs, stop certolizumab and begin anti-viral therapy.
- Demyelinating disease, exacerbation or new onset, may occur.
- Cytopenias and pancytopenia may develop. Advise patients to seek immediate medical attention if symptoms develop, and consider stopping certolizumab.
- Lupus-like syndrome—stop certolizumab if the syndrome develops.
Commentary: The FDA approval was based on a multi-center, clinical trial, which showed that patients treated with 200 mg certolizumab every other week demonstrated greater reduction in radiographic progression compared with placebo-treated patients at Week 24.The most common adverse reactions (≥7%) are upper respiratory tract infection, rash and urinary tract infection.
Golimumab (Simponi):19 Injection
Drug class: TNF inhibitor
Boxed warning: Refer to *ISI (p. 17)
Warnings & Precautions
- Do not start golimumab during an active infection. If an infection develops, monitor carefully, and stop golimumab if the infection becomes serious.
- For patients who develop a systemic illness on golimumab, consider empiric antifungal therapy for those who reside in or travel to regions where mycoses are endemic.
- HBV many occur. Monitor HBV carriers during and for several months after therapy. If reactivation occurs, stop golimumab and begin anti-viral therapy.
- The incidence of lymphoma was seen more often than in the general U.S. population. Cases of other malignancies have been observed among patients receiving TNFi.
- Heart failure, worsening or new onset, may occur. Stop golimumab if new or worsening symptoms occur.
- Demyelinating disease, exacerbation or new onset, may occur.
- Serious systemic hypersensitivity reactions, including anaphylaxis, may occur.
Commentary: The FDA approval of golimumab is based on results from a clinical trial that demonstrated a higher proportion of patients having significant improvement in the signs and symptoms of PsA. The most common adverse reactions (≥5%) are upper respiratory tract infection and nasopharyngitis.
Abatacept (Orencia):20 Injection/Infusion
Drug class: selective T cell costimulation modulator, DMARD, immunomodulator
Warnings & Precautions
- Concomitant use with a TNF blocker can increase the risk of (serious) infections. Discontinue abatacept if a serious infection occurs.
- Anaphylaxis or anaphylactoid reactions can occur after the first infusion and can be life threatening. Appropriate medical support should be immediately available in the event of a reaction. Post-marketing experience notes at least one case of fatal anaphylaxis following the first abatacept infusion. Following an anaphylactic or other serious allergic reaction, abatacept administration should be stopped immediately, with appropriate therapy instituted. Abatacept should be permanently discontinued.
- Patients with a history of recurrent infections or underlying conditions predisposing them to infections may experience more infections.
- Screen for latent TB prior to initiating therapy. Patients testing positive should be treated prior to initiating abatacept.
- Live vaccines should not be given concurrently or within three months of abatacept discontinuation.
- Based on its mechanism of action, abatacept may blunt the effectiveness of some immunizations.
- Chronic obstructive pulmonary disease (COPD) patients may develop more frequent adverse respiratory events.
Commentary: The FDA approved abatacept based on the results of two randomized, controlled trials that involved nearly 600 adults with long-standing PsA. The most common adverse reactions (≥10%) are headache, upper respiratory tract infection, nasopharyngitis and nausea.
Ustekinumab (Stelara):21 Injection
Drug class: monoclonal antibody, interleukin (IL-12/23) inhibitor
Warnings & Precautions
- Serious infections have occurred. Do not start ustekinumab during any clinically important active infection. If a serious infection or clinically significant infection develops, consider discontinuing ustekinumab until the infection resolves.
- Theoretical infection risk—Serious infections from mycobacteria, salmonella and Bacillus Calmette-Guerin (BCG) vaccinations have occurred in patients genetically deficient in IL-12/23. Diagnostic tests for these infections should be considered as dictated by clinical circumstances.
- Evaluate patients for TB prior to initiating treatment with ustekinumab. Initiate treatment of latent TB before administering ustekinumab.
- Ustekinumab may increase malignancy risk. The safety of using ustekinumab in patients with a history of, or a known, malignancy has not been evaluated.
- Anaphylaxis or other clinically significant hypersensitivity reactions may occur.
- Reversible posterior leukoencephalopathy syndrome (RPLS) has been reported in one case. If suspected, treat promptly and discontinue ustekinumab.
- Cases of noninfectious, interstitial pneumonia, eosinophilic pneumonia and cryptogenic organizing pneumonia have been reported during post-
marketing ustekinumab use. If diagnosis is confirmed, discontinue ustekinumab and initiate appropriate treatment.
Commentary: The FDA based its approval on two randomized, controlled trials of more than 900 patients. The study participants had at least five tender and swollen joints and high levels of C-reactive protein (a measure of inflammation), and were not responding to current therapies. The most common adverse reactions (≥3%) are arthralgia and nausea.
Tofacitinib (Xeljanz/ Xeljanz XR):22 Tablets
Drug class: DMARD, JAK inhibitor
Boxed warning: Refer to *ISI (p. 17) and
- Lymphoma and other malignancies have been observed in tofacitinib-treated patients. Epstein-Barr virus-associated post-transplant lymphoproliferative disorder has been observed at an increased rate in renal transplant patients treated with tofacitinib and concomitant immunosuppressive medications.
Warnings & Precautions
- Avoid use of tofacitinib during an active serious infection, including localized infections.
- Use with caution in patients who may be at increased risk for gastrointestinal perforations.
- Monitor laboratory parameters: lymphocytes, neutrophils, hemoglobin, liver enzymes and lipids, due to potential changes.
- Avoid use of live vaccines with tofacitinib.
Commentary: Tofacitinib is the first and only drug of a new class of treatments called JAK inhibitors approved for the treatment of PsA. The FDA approved tofacitinib for adults with active PsA who have had an inadequate response or intolerance to methotrexate or other DMARDs. The most common adverse reactions (≥2%) are upper respiratory tract infection, nasopharyngitis, diarrhea and headache.
Brodalumab (Siliq):23 Injection
Important note: The manufacturer has completed phase 3 testing of brodalumab for psoriatic arthritis, and the drug was included in the ACR/NPF guideline. However, it is not currently approved by the FDA for this indication.
Drug class: monoclonal antibody, interleukin 17 receptor antagonist (IL17-RA)
Boxed warning: Suicidal ideation and behavior
- Suicidal ideation and behavior, including completed suicides, have occurred in patients treated with brodalumab.
- Prior to prescribing, weigh potential risks and benefits in patients with a history of depression and/or suicidal ideation or behavior.
- Patients with new or worsening suicidal thoughts and behavior should be referred to a mental health professional.
- Advise patients and caregivers to seek medical attention for manifestations of suicidal ideation or behavior, new onset or worsening depression, anxiety, or other mood changes.
- Brodalumab is available only through a restricted program called the SILIQ REMS Program.
Warnings & Precautions
- Serious infections have occurred. Consider the risks and benefits prior to initiating brodalumab in patients with a chronic infection or a history of recurrent infection. If a serious infection develops, discontinue brodalumab until the infection resolves.
- Evaluate patients for TB prior to initiating treatment with brodalumab.
- Crohn’s disease occurred in brodalumab-
treated patients during clinical trials. Discontinue brodalumab if this develops.
- Avoid using live vaccines concurrently with brodalumab.
Commentary: The most common adverse reactions (≥3%) are arthralgia and nausea.
Ixekizumab (Taltz):24 Injection
Drug class: monoclonal antibody, IL-17RA
Warnings & Precautions
- Serious infections have occurred. Instruct patients to seek medical advice if signs or symptoms of a clinically important chronic or acute infection occur. If a serious infection develops, discontinue ixekizumab until the infection resolves.
- Evaluate for TB prior to initiating treatment.
- If a serious hypersensitivity reaction occurs, discontinue ixekizumab immediately and initiate appropriate therapy.
- IBD, including exacerbations, occurred during clinical trials. Patients who are treated with ixekizumab and have IBD should be monitored closely.
Commentary: The FDA approval of ixekizumab for active PsA was based on two randomized, controlled clinical trials that included more than 670 adult patients. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea and tinea infections.
Secukinumab (Cosentyx):25 Injection
Drug class: Monoclonal antibody, IL-17 RA
Warnings & Precautions
- Serious infections have occurred. Caution should be exercised when considering using secukinumab for patients with a chronic infection or a history of recurrent infection. If a serious infection develops, discontinue the drug until the infection resolves.
- Prior to initiating treatment with secukinumab, evaluate for TB.
- Caution should be exercised when prescribing secukinumab to patients with IBD; cases of IBD were observed in clinical trials.
- If an anaphylactic reaction or other serious hypersensitivity reaction occurs, discontinue secukinumab immediately and initiate appropriate therapy.
Commentary: A new study (analysis of results from an earlier clinical trial) further confirms the efficacy of secukinumab.26 The study uses a new assessment tool called Psoriatic Arthritis Disease Activity Score (PASDAS), which is more comprehensive than other evaluation tools. According to the new analysis, PsA patients who used secukinumab were more likely to achieve remission or low disease activity, as defined by PASDAS, after 16 weeks than those using a placebo. They were also more likely to stay in remission or low disease activity states for a two-week period. The most common adverse reactions (≥1%) are nasopharyngitis, diarrhea and upper respiratory tract infection.
Mary Choy, PharmD, BCGP, FASHP, is a medical writer and editor living in New York City. Dr. Choy is director of pharmacy practice at the New York State Council of Health-System Pharmacists.
References
- Singh JA, Guyatt G, Ogdie A, et al. 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Rheumatol. 2019 Jan;71(1):5–32.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015 Jun;74(6):1045–1050.
- Lebowohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US perspectives in the management of psoriasis and psoriatic arthritis: Patient and physician results from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Am J Clin Dermatol. 2016 Feb;17(1): 87–97.
- Araujo EG, Finzel S, Englbrecht M, et al. High incidence of disease recurrence after discontinuation of disease-modifying antirheumatic drug treatment in patients with psoriatic arthritis in remission. Ann Rheum Dis. 2015 Apr;74(4):655–660.
- Zang HA, Ndosi M, Adams J, et al. EULAR recommendations for patient education for people with inflammatory arthritis. Ann Rheum Dis. 2015 Jun;74(6):954–962.
- The National Psoriasis Foundation. Financial assistance resource center.
- FDA website. Otezla prescribing information. 2017 Jun.
- FDA website. Enbrel prescribing information. 2018 May.
- FDA website. Erelzi prescribing information. 2016 Aug.
- FDA website. Remicade prescribing information. 2018 Jun.
- FDA website. Inflectra prescribing information. 2018 Jul.
- FDA website. Renflexis prescribing information. 2019 Mar.
- FDA website. Ixifi prescribing information. 2017 Dec.
- FDA website. Humira prescribing information. 2018 Dec.
- FDA website. Amjevita prescribing information. 2018 Mar.
- FDA website. Cyltezo prescribing information. 2017 Aug.
- FDA website. Hyrimoz prescribing information. 2018 Oct.
- FDA website. Cimzia prescribing information. 2019 Feb.
- FDA website. Simponi prescribing information. 2018 Mar.
- FDA website. Orencia prescribing information. 2017 Dec.
- FDA website. Stelara prescribing information. 2018 Jun.
- FDA website. Xeljanz prescribing information. 2018 May.
- FDA website. Siliq prescribing information. 2017 Feb.
- FDA website. Taltz prescribing information. 2018 May.
- FDA website. Cosentyx prescribing information. 2018 Jan.
- Coates LC, Gladman DD, Nash P, et al. Secukinumab provides sustained PASDAS-defined remission in psoriatic arthritis and improves health-related quality of life in patients achieving remission: 2-year results from the phase III FUTURE 2 study. Arthritis Res Ther. 2018 Dec;20(1):272.