Over the past few years, biosimilars and other new drugs have been introduced to treat rheumatic illnesses. Some of the conditions we treat have numerous drug option; others have few or only off-label options. This series, “Rheumatology Drugs at a Glance,” provides streamlined information on the administration of biologic, biosimilar and other medications used to treat patients with rheumatic disease. In Part 2, we discuss the 17 available options to treat psoriasis (see Table 1).
Psoriasis vulgaris (the medical name for the most common form of psoriasis) is a chronic inflammatory skin disease that manifests characteristic plaques on the skin: well-demarcated, red plaques with silvery scales that can appear on any area of the skin, although the scalp, elbows, knees and trunk are the most common locations. Although skin involvement is often the most prominent and widely recognized manifestation of this disease, recognition of the condition as a chronic, multisystem inflammatory disorder is imperative to optimize management.1 Psoriasis follows a relapsing course and can change unpredictably over time in individual patients, which can have a significant effect on their quality of life. Addressing both physical and psychosocial aspects of psoriasis is necessary for optimal disease management.
The majority of patients with mild to moderate psoriasis can manage their disease with topical medications or phototherapy. However, patients with moderate to severe disease require treatment with biologic agents, either as monotherapy or combined with other topical or systemic medications.
An autoimmune disease, psoriasis primarily affects the skin, but is associated with the inflammatory arthritis, psoriatic arthritis (PsA). PsA has a prevalence of 25–30% in patients with psoriasis.2
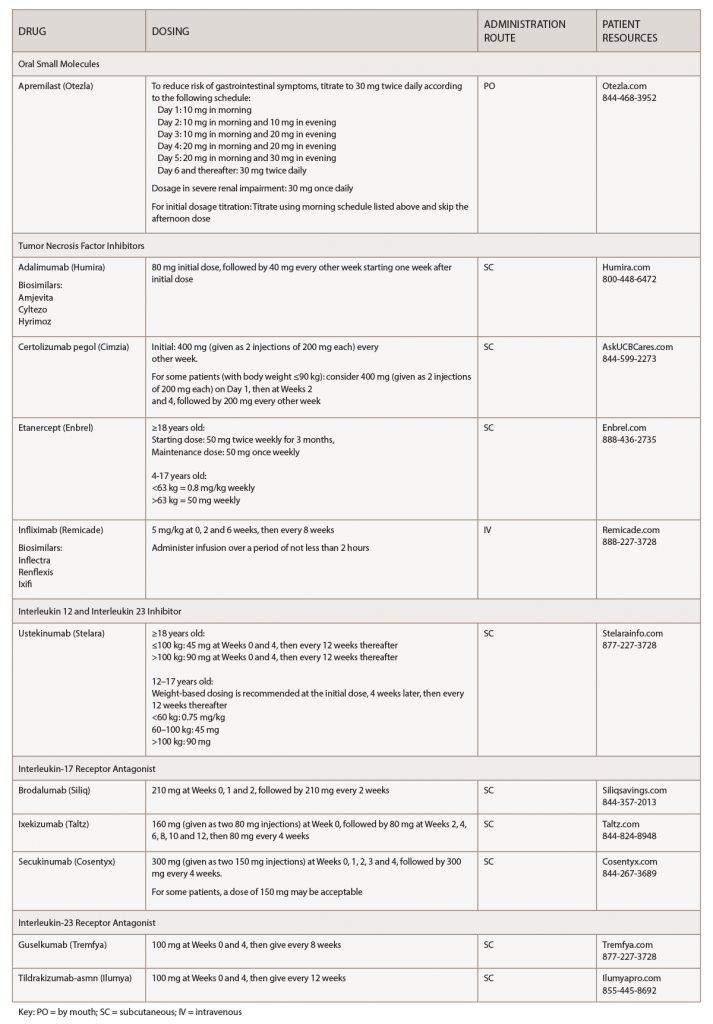
(click for larger image) Table 1: Psoriasis Medications at a Glance
Note: These medications should be used for adults 18 years old or older, unless otherwise specified.
Skin conditions carry a stigma, and psoriasis is associated with a major psychological burden.3 Adherence to biologics is a major barrier for patients, and biologic use is associated with high discontinuation rates.4 When treating patients with psoriasis, it’s important to explain the natural history of the disease, and also to advise patients that this is a chronic disease that can be controlled with therapy, but that remission does not equal cure.
Educating patients and improving communication will optimize the patient-provider relationship through shared decision making. It’s important to select the best therapeutic option for, and with, the patient to improve adherence. This will positively impact patient satisfaction.
Biologic therapies for psoriasis offer patients improved quality of life over traditional systemic therapy with regard to improved efficacy and less toxicity.5 Providers should be aware of the major risks when using biologics for the treatment of psoriasis: infection and nonmelanoma skin cancer. Appropriate counseling and screening are necessary to attenuate the risk.
All biologic prescriptions include an FDA-approved medication guide for patient instruction. Patients should be encouraged to read this before starting a biologic and after each refill.
Treatments for psoriasis can be expensive, and healthcare providers can help make treatment costs more manageable for patients. A number of financial assistance resources are available to patients, and patients can also contact the manufacturers to request copay assistance. Your staff can explore patient assistance programs for those who don’t have insurance.6
Depending on a patient’s coverage and the medication(s) they use, a specialty pharmacy may dispense the drugs. Most specialty pharmacies have clinical pharmacists available to help patients manage insurance issues; monitor for adverse effects, effectiveness and adherence issues; and serve as a drug information resource.
Guidelines
For additional information, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) have updated psoriasis treatment guidelines. Their first joint guideline is for managing and treating psoriasis with biologics. It can be reviewed here.1 The AAD/NPF team has also published a second guideline on treating psoriasis with awareness and attention to comorbidities.7
The new psoriasis guidelines arrive on the heels of the new PsA treatment guideline, which was discussed in Part 1 of this series.8 These new guidelines mark a new era in the treatment of psoriatic disease.
Apremilast (Otezla):9 tablets
Drug class: DMARD, phosphodiesterase 4 (PDE4) inhibitor
Warnings & Precautions
- Depression: Individuals should be alerted to watch for the emergence or worsening of depression, suicidal thoughts or other mood changes. If these changes occur, they should contact their physician. Carefully weigh the risks and
benefits of apremilast treatment in patients with a history of depression and/or suicidal thoughts or behavior. - Weight decrease: Weight should be regularly monitored. If unexplained or clinically significant weight loss occurs, consider discontinuing apremilast.
- Drug interactions: Using apremilast with strong cytochrome P450 (CYP 450) enzyme inducers (e.g., rifampin, phenobarbital, carbamazepine, phenytoin) is not recommended due to potential loss of efficacy.
Dosage & Administration
To reduce the risk of gastrointestinal symptoms, titrate to 30 mg twice daily according to the following schedule:
- Day 1: 10 mg in the morning
- Day 2: 10 mg in the morning and 10 mg in the evening
- Day 3: 10 mg in morning and 20 mg in the evening
- Day 4: 20 mg in the morning and 20 mg in the evening
- Day 5: 20 mg in the morning and 30 mg in the evening
- Day 6 and thereafter: 30 mg twice daily
Dosage in severe renal impairment: 30 mg once daily. For initial dosage titration: Titrate using the morning schedule listed above and skip the afternoon dose.
Commentary: The safety and effectiveness of apremilast were evaluated in two multicenter clinical trials, which enrolled a total of 1,257 patients. Patients treated with the drug experienced significant, clinical improvement in plaque psoriasis at Week 16 of the studies as measured by the Psoriasis Area and Severity Index (PASI 75) score. The most common adverse reactions (≥5%) are diarrhea, nausea and headache.
*Important Safety Information (ISI)
This ISI is applicable to all tumor necrosis factor inhibitors (TNFi’s) and some other immune modulators.
Serious Infections & Malignancies
- There is an increased risk of serious infections leading to hospitalization or death, including tuberculosis (TB), bacterial sepsis, invasive fungal infections (e.g., histoplasmosis) and infections due to other opportunistic pathogens. If these develop, discontinue the drug.
- Patients should be tested for latent TB prior to starting the drug and during therapy. Treatment for latent TB should be initiated prior to starting the drug. Patients, including those who tested negative for latent TB infection prior to initiating therapy, should be closely monitored for the development of signs and symptoms of infection during and after treatment with the drug. Induration of 5 mm or greater with tuberculin skin testing should be considered a positive test result when assessing if treatment for latent tuberculosis is needed prior to initiating the drug, even for patients previously vaccinated with Bacile Calmette-Guerin (BCG).
- Lymphoma and other malignancies, some fatal, have been reported in children and adolescents treated with TNFi’s.
Adalimumab (Humira):10 injection
Biosimilars: Adalimumab-atto (Amjevita),11 Adalimumab-adbm (Cyltezo),12 Adalimumab-adaz (Hyrimoz)13
Drug class: DMARD, TNFi
Boxed warning: Refer to *ISI (above) and
- Fatal hepatosplenic T cell lymphoma (HSTCL) has occurred postmarketing in adolescent and young adult inflammatory bowel disease (IBD) patients treated with TNFi’s.
Warnings & Precautions
- Do not start adalimumab during an active infection. If an infection develops, monitor carefully, and stop adalimumab if the infection becomes serious.
- Invasive fungal infections—for patients who develop a systemic illness on adalimumab, consider empiric anti-fungal therapy for those who reside in or travel to regions where mycoses are endemic.
- The incidence of malignancies was greater in adalimumab-treated patients than in the control groups.
- Anaphylaxis or serious allergic reactions may occur.
- Hepatitis B virus (HBV) reactivation can occur. Monitor HBV carriers during and several months after therapy. If reactivation occurs, stop adalimumab and begin anti-viral therapy.
- Demyelinating disease exacerbation, or new onset, may occur.
- Cytopenias, pancytopenia—advise patients to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (e.g., persistent fever, bruising, bleeding, pallor) while on adalimumab. Consider discontinuation of therapy in patients with confirmed significant hematologic abnormalities.
- Heart failure, worsening or new onset, may occur.
- Lupus-like syndrome—stop adalimumab if syndrome develops.
Dosage & Administration
Adalimumab is administered by subcutaneous injection. The recommended dose is an initial dose of 80 mg, followed by 40 mg every other week starting one week after the initial dose.
Commentary: The approval of adalimumab for plaque psoriasis was based on two pivotal clinical trials that had enrolled more than 1,400 patients. Nearly three in four patients achieved at least a PASI 75 at Week 16 of treatment vs. placebo. The most common adverse reactions (≥10%) are infections (e.g., upper respiratory, sinusitis), injection-site reactions, headache and rash.
Certolizumab pegol (Cimzia):14 injection
Drug class: DMARD, TNFi
Boxed warning: Refer to *ISI (above)
Warnings & Precautions
- Do not start certolizumab during an active infection. If an infection develops, monitor carefully, and stop certolizumab if the infection becomes serious.
- Invasive fungal infections—for patients who develop a systemic illness on certolizumab, consider empiric anti-fungal therapy for those who reside in or travel to regions where mycoses are endemic.
- Cases of lymphoma and other malignancies have been observed in patients receiving TNFi’s.
- Heart failure, worsening or new onset, may occur.
- Anaphylaxis or serious allergic reactions may occur.
- HBV reactivation can occur. Test for HBV infection before starting certolizumab. Monitor HBV carriers during and several months after therapy. If reactivation occurs, stop certolizumab and begin anti-viral therapy.
- Demyelinating disease, exacerbation or new onset, may occur.
- Cytopenias, pancytopenia—advise patients to seek immediate medical attention if symptoms develop, and consider stopping certolizumab.
- Lupus-like syndrome—stop certolizumab if syndrome develops.
Dosage & Administration
Certolizumab is administered by subcutaneous injection. The recommended initial dose is 400 mg (given as two subcutaneous injections of 200 mg) every other week. For some patients (with body weight ≤90 kg), a dose of 400 mg (given as two subcutaneous injections of 200 mg each) initially and at Weeks 2 and 4, followed by 200 mg every other week may be considered.
Commentary: The U.S. Food and Drug Administration (FDA) approval was based on three clinical trials that enrolled over 1,000 patients. The results showed that patients had significant improvement in psoriasis symptoms through Week 48 in adults who received certolizumab compared with placebo. The most common adverse reactions (≥7%) are upper respiratory tract infection, rash and urinary tract infection.
Etanercept (Enbrel):15 injection
Drug class: DMARD, TNFi
Boxed warning: Refer to *ISI (left)
Warnings & Precautions
- Do not start etanercept during an active infection. If an infection develops, monitor carefully, and stop etanercept if the infection becomes serious.
- Consider empiric anti-fungal therapy for patients at risk (those who reside in or travel to regions where mycoses are endemic) for invasive fungal infections who develop a severe systemic illness on etanercept.
- Demyelinating disease, exacerbation or new onset, may occur.
- Cases of lymphoma have been observed in patients receiving TNFi’s.
- Congestive heart failure, worsening or new onset, may occur.
- Advise patients to seek immediate medical attention if symptoms of pancytopenia or aplastic anemia develop, and consider stopping etanercept.
- Monitor HBV carriers for reactivation during and following therapy. If reactivation occurs, consider stopping etanercept and beginning anti-viral therapy.
- Anaphylaxis or serious allergic reactions may occur.
- Stop etanercept if lupus-like syndrome or autoimmune hepatitis develops.
Dosage & Administration
Etanercept is administered by subcutaneous injection.
Adults: The starting dose is 50 mg twice weekly for three months. The maintenance dose is 50 mg once weekly.
Pediatrics:
- 63 kg (138 lbs.) or more: the recommended dose is 50 mg weekly.
- Less than 63 kg (138 lbs.): the recommended dose is 0.8 mg/kg weekly.
Commentary: Etanercept is approved for adult and pediatric plaque psoriasis. It is the first and only systemic therapy to treat children aged 4-17 years for chronic moderate to severe plaque psoriasis. The most common adverse reactions (≥5%) are infections and injection-site reactions.
Infliximab (Remicade):16 infusion
Biosimilar(s): Infliximab-dyyb (Inflectra),17 Infliximab-abda (Renflexis),18 Infliximab-qbtx (Ixifi)19
Drug class: DMARD, TNFi
Boxed warning: Refer to *ISI (above) and
- Fatal hepatosplenic T-cell lymphoma (HSTCL) have been reported in patients treated with TNFi’s, postmarketing. All infliximab cases occurred in IBD patients, who were mostly adolescent or young adult males. All had received azathioprine or 6-mercaptopurine concomitantly with infliximab at or prior to diagnosis.
Warnings & Precautions
- Do not give infliximab during an active infection. If an infection develops, monitor carefully and stop infliximab if the infection becomes serious.
- Invasive fungal infections—for patients who develop a systemic illness on infliximab, consider empiric anti-fungal therapy for those who reside or travel to regions where mycoses are endemic
- The incidence of malignancies, including lymphoma, was greater in infliximab-treated patients than in controls. Due to the risk of HSTCL, carefully assess the risk/benefit especially in IBD patients, in males, and if receiving azathioprine or 6-mercaptopurine treatment.
- HBV reactivation can occur. Test for HBV infection before starting infliximab. Monitor HBV carriers during and several months after therapy. If reactivation occurs, stop infliximab and begin anti-viral therapy.
- Hepatotoxicity—rare severe hepatic reactions, some fatal or necessitating liver transplantation have occurred. Stop infliximab in cases of jaundice and/or marked liver enzyme elevations.
- Heart failure, new onset or worsening symptoms, may occur.
- Cytopenias—advise patients to seek immediate medical attention if signs and symptoms develop, and consider stopping infliximab.
- Hypersensitivity—serious infusion reactions including anaphylaxis or serum sickness-like reactions may occur.
- Demyelinating disease, exacerbation or new onset, may occur.
- Lupus-like syndrome—stop infliximab if syndrome develops.
- Live vaccines or therapeutic infectious agents should not be given with infliximab.
Dosage & Administration
Infliximab is administered by intravenous infusion over a period of not less than two hours. The recommended dose is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks, followed by a maintenance regimen of 5 mg/kg every eight weeks.
Commentary: The FDA approved infliximab for treatment of psoriasis based on data from two multicenter clinical trials that enrolled 1,200 patients. The results showed that a majority of infliximab-treated patients achieved clinically significant levels of skin clearance with induction and every-eight-week maintenance therapy. The most common adverse reactions (≥10%) are infections (e.g., upper respiratory, sinusitis, pharyngitis), infusion-related reactions, headache and abdominal pain.
Ustekinumab (Stelara):20 injection
Drug class: Monoclonal antibody, interleukin (IL) 12/23) inhibitor
Warnings & Precautions
- Serious infections have occurred. Do not start ustekinumab during any clinically important active infection. If a serious infection or clinically significant infection develops, consider discontinuing ustekinumab until the infection resolves.
- Theoretical infection risk: Serious infections from mycobacteria, salmonella and Bacillus Calmette-Guerin (BCG) vaccinations have occurred in patients genetically deficient in IL-12/IL-23. Diagnostic tests for these infections should be considered as dictated by clinical circumstances.
- Evaluate patients for TB prior to initiating treatment with ustekinumab. Initiate treatment of latent TB before administering ustekinumab.
- Ustekinumab may increase malignancy risk. The safety of using ustekinumab in patients with a history of, or a known, malignancy has not been evaluated.
- Anaphylaxis or other clinically significant hypersensitivity reactions may occur.
- Reversible posterior leukoencephalopathy syndrome (RPLS) has been reported in one case. If suspected, treat promptly and discontinue ustekinumab.
- Cases of noninfectious, interstitial pneumonia, eosinophilic pneumonia and cryptogenic organizing pneumonia have been reported during postmarketing ustekinumab use. If diagnosis is confirmed, discontinue ustekinumab and initiate appropriate treatment.
Dosage & Administration
Subcutaneous Adult Dosage Regimen:
- For patients weighing 100 kg or less, the recommended dose is 45 mg initially and four weeks later, followed by 45 mg every 12 weeks.
- For patients weighing more than 100 kg, the recommended dose is 90 mg initially and four weeks later, followed by 90 mg every 12 weeks.
Subcutaneous Adolescent Dosage Regimen:
Administer the drug subcutaneously at Weeks 0 and 4, then every 12 weeks thereafter.
The recommended dose for adolescents (12–17 years old) is based on body weight.
- Less than 60 kg: the recommended dose is 0.75 mg/kg.
- 60 kg to 100 kg: the recommended dose is 45 mg.
- More than 100 kg: the recommended dose is 90 mg.
Commentary: The FDA approved ustekinumab for moderate to severe plaque psoriasis treatment in adults based on data from two randomized controlled studies, and in adolescents based on data from one randomized controlled study. The PASI 75 results were both clinically and statistically significant at the end of the study. The most common adverse reactions (≥3%) are arthralgias and nausea.
Brodalumab (Siliq):21 injection
Drug class: Monoclonal antibody, IL-17 receptor antagonist (IL-17-RA)
Boxed warning: Suicidal ideation and behavior
- Suicidal ideation and behavior, including completed suicides, have occurred in patients treated with brodalumab.
- Prior to prescribing, weigh potential risks and benefits in patients with a history of depression and/or suicidal ideation or behavior.
- Patients with new or worsening suicidal thoughts and behavior should be referred to a mental health professional.
- Advise patients and caregivers to seek medical attention for manifestations of suicidal ideation or behavior, new onset or worsening depression, anxiety, or other mood changes.
- Brodalumab is available only through a restricted program called the SILIQ Risk Evaluation and Mitigation Strategy (REMS) Program.
Warnings & Precautions
- Serious infections have occurred. Consider the risks and benefits prior to initiating brodalumab in patients with a chronic infection or a history of recurrent infection. If a serious infection develops, discontinue brodalumab until the infection resolves.
- Evaluate patients for TB prior to initiating treatment with brodalumab.
- Crohn’s disease occurred in brodalumab-treated patients during clinical trials. Discontinue brodalumab if this develops.
- Avoid using live vaccines concurrently with brodalumab.
Dosage & Administration
The recommended dose is 210 mg administered by subcutaneous injection at Weeks 0, 1, and 2 followed by 210 mg every two weeks. If an adequate response has not been achieved after 12 to 16 weeks of treatment, consider discontinuing therapy. Continued treatment beyond 16 weeks in patients who have not achieved an adequate response is not likely to result in greater success.
Commentary: Advise the patient to read the FDA-approved medication guide before starting to use brodalumab and each time the prescription is renewed, because important new information may have been added. The most common adverse reactions (≥3%) are arthralgias and nausea.
Ixekizumab (Taltz):22 injection
Drug class: Monoclonal antibody, IL-17-RA
Warnings & Precautions
- Serious infections have occurred. Instruct patients to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a serious infection develops, discontinue ixekizumab until the infection resolves.
- Evaluate for TB prior to initiating treatment.
- If a serious hypersensitivity reaction occurs, discontinue ixekizumab immediately and initiate appropriate therapy.
- IBD, including exacerbations, occurred during clinical trials. Monitor closely patients who are treated with ixekizumab and have IBD.
Dosage & Administration
Ixekizumab is administered by subcutaneous injection. The recommended dose is 160 mg (two 80 mg injections) at Week 0; followed by 80 mg at Weeks 2, 4, 6, 8, 10 and 12; then 80 mg every four weeks.
Commentary: The FDA approval of ixekizumab for psoriasis was based on three randomized, controlled clinical trials that included a total of 3,866 adult patients. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea and tinea infections.
Secukinumab (Cosentyx):23 injection
Drug class: Monoclonal antibody, IL-17-RA
Warnings & Precautions
- Serious infections have occurred. Caution should be exercised when considering using secukinumab in patients with a chronic infection or a history of recurrent infection. If a serious infection develops, discontinue the drug until the infection resolves.
- Prior to initiating treatment with secukinumab, evaluate for TB.
- Caution should be exercised when prescribing secukinumab to patients with IBD; cases of IBD were observed in clinical trials.
- If an anaphylactic reaction or other serious hypersensitivity reaction occurs, discontinue secukinumab immediately and initiate appropriate therapy.
Dosage & Administration
The recommended dose is 300 mg by subcutaneous injection at Weeks 0, 1, 2, 3, and 4, followed by 300 mg every four weeks. Each 300 mg dosage is given as two subcutaneous injections of 150 mg. For some patients, a dosage of 150 mg may be acceptable.
Commentary: Clinical studies with secukinumab showed that patients had a 67–87% PASI 75 response. At Week 12, 81% of those patients had maintained PASI 75, an improvement maintained after one year. The most common adverse reactions (≥1%) are nasopharyngitis, diarrhea and upper respiratory tract infection.
Guselkumab (Tremfya):24 injection
Drug class: Monoclonal antibody, IL-23-RA
Warnings & Precautions
- Serious infections have occurred. Guselkumab may increase the risk of infection. Instruct patients to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a serious infection develops, discontinue the drug until the infection resolves.
- Prior to initiating treatment with secukinumab, evaluate for TB.
Dosage & Administration
Guselkumab is administered by subcutaneous injection. The recommended dose is 100 mg at Week 0, Week 4, and every eight weeks thereafter.
Commentary: Guselkumab was the first biologic approved that selectively blocks IL-23. It received FDA approval based on results from a clinical development program that included more than 2,000 patients. The most common adverse reactions (≥1%) are upper respiratory infections, headache, injection site reactions, arthralgias, diarrhea, gastroenteritis, tinea infections and herpes simplex infections.
Tildrakizumab-asmn (Ilumya):25 injection
Drug class: Monoclonal antibody, IL-23-RA
Warnings & Precautions
- Hypersensitivity: If a serious allergic reaction occurs, discontinue tildrakizumab-asmn immediately and initiate appropriate therapy.
- Serious infections have occurred. Guselkumab may increase the risk of infection. Instruct patients to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a serious infection develops, discontinue the drug until the infection resolves.
- Prior to initiating treatment with tildrakizumab-asmn, evaluate for TB.
Dosage & Administration
Tildrakizumab-asmn is administered by subcutaneous injection. The recommended dose is 100 mg at Weeks 0 and 4, and every 12 weeks thereafter.
Commentary: The FDA approval was based on results from two clinical trials that enrolled 926 patients. There was significant improvement in patients who received tildrakizumab-asmn 100 mg compared with placebo. The most common adverse reactions (≥1%) are upper respiratory infections, injection site reactions, and diarrhea.
Mary Choy, PharmD, BCGP, FASHP, is a medical writer and editor living in New York City. She is the director of pharmacy practice for the New York State Council of Health-System Pharmacists.
References
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019 Apr;80(4):1029–1072.
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013 Nov;69(5):729–735.
- Patryk K, Marcinkiewicz K, Bergler-Czop B, et al. How does stigma affect people with psoriasis? Postepy Dermatol Alergol. 2017 Feb;34(1):36–41.
- Zschocke I, Ortland C, Reich K. Evaluation of adherence predictors for the treatment of moderate to severe psoriasis with biologics: the importance of physician-patient interaction and communication. J Eur Acad Dermatol Venereol. 2017 Jun;31(6):1014:1020.
- Poelman S, Keeling CP, Metelitsa AI, et al. Practical guidelines for managing patients with psoriasis on biologics: an update. J Cutan Med Surg. 2019 Jan/Feb;23(1_suppl):3S–12S.
- The National Psoriasis Foundation. Financial assistance resource center.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019 Apr;80(4):1073–1113.
- Singh JA, Guyatt G, Ogdie A, et al. 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Rheumatol. 2019 Jan;71(1):5–32.
- FDA website. Otezla prescribing information. 2017 Jun.
- FDA website. Humira prescribing information. 2018 Dec.
- FDA website. Amjevita prescribing information. 2018 Mar.
- FDA website. Cyltezo prescribing information. 2017 Aug.
- FDA website. Hyrimoz prescribing information. 2018 Oct.
- FDA website. Cimzia prescribing information. 2019 Feb.
- FDA website. Enbrel prescribing information. 2018 May.
- FDA website. Remicade prescribing information. 2018 Jun.
- FDA website. Inflectra prescribing information. 2018 Jul.
- FDA website. Renflexis prescribing information. 2019 Mar.
- FDA website. Ixifi prescribing information. 2017 Dec.
- FDA website. Stelara prescribing information. 2018 Jun.
- FDA website. Siliq prescribing information. 2017 Feb.
- FDA website. Taltz prescribing information. 2018 May.
- FDA website. Cosentyx prescribing information. 2018 Jan.
- FDA website. Tremfya prescribing information. 2019 Jan.
- FDA website. Ilumya prescribing information. 2018 Mar.



