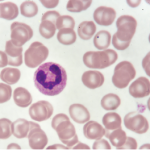
Image Credit: Gary Carlson/ScienceSource.com
Activated neutrophils.
SAN FRANCISCO—To unravel the mysteries of how autoimmunity begins in the body and, one day, to interrupt that process, rheumatology researchers are exploring the role of neutrophils, especially when they form and release neutrophil extracellular traps (NETs).
At a panel discussion on Nov. 6, 2015, held at the American College of Rheumatology’s Basic Research Conference, the precursor to the 2015 ACR/ARHP Annual Meeting, three speakers explored the emerging importance of studying the role of NETs in autoimmunity in such diseases as systemic lupus erythematosus, vasculitis, rheumatoid arthritis and gout.
Neutrophils are a first-line defense system against infectious agents. When they release chromatin and granular proteins to form NETs, they can trap and destroy pathogens as a last line of defense against disease. However, neutrophils and NETs may also play a role in the induction of the autoimmune response, a connection that is not yet fully understood, the panelists said.
Neutrophils and their cell death, called NETosis, play a role in lupus pathogenesis, said Mariana J. Kaplan, MD, chief of the Systemic Autoimmunity Branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) in Bethesda, Md.
Low-density granulocytes (LDGs), a subset of neutrophils, are present in the peripheral blood mononuclear cells in samples from lupus patients. “Neutrophils are the main source of type-1 interferon in lupus bone marrow,” she said. “They are prematurely released from marrow due to some stimuli, although we don’t yet know what.”
LDGs express high levels of the antigen CD-15, have a great ability to synthesize type-1 interferon, and are thought to play a role in SLE pathogenesis and organ damage, she said.
“We hypothesize that LDGs may play important pathogenic roles in lupus due to their enhanced pro-inflammatory phenotype, increased capacity to form NETs and damage the endothelium,” Dr. Kaplan said in a follow-up interview.
Although most lupus patients show an enhanced ability to form NETs, about one-third have an impaired ability to clear NETs from their blood. “This imbalance may promote enhanced exposure of modified auto-antigens that could promote autoimmune responses,” she said. NETs amplify type-1 interferon responses and activate complement in these patients, she said. NETs are also a strong activator of macrophages and can exacerbate inflammatory responses in the periphery or affected organs.
Lupus LDG NETs are enriched in oxidized, interferogenic, mitochondrial DNA, Dr. Kaplan said. “Not all NETs are created equal. NETs are quite toxic to vasculature, or vasculopathic. They’re good at killing endothelial cells, and they’re potent inducers of thrombosis.” NETs also oxidize lipoproteins and are proatherogenic, and so may play a role in cardiovascular disease risk for these patients.